Unlocking the world through monitoring
by Niels Kruize, CCO, MolGen
“Automation and industrialization of COVID-19 PCR-testing will facilitate an immense scale-up of the global PCR-testing capacity. It will allow for a new approach to keep society open: preventive, regular testing (twice per week), which will create green bubbles and get us ahead of the curve” – Niels Kruize, CCO, MolGen
Why do we need PCR testing for monitoring?
To get ahead of the curve, we will need to change our methodology of COVID-19 testing. After two years, the pandemic continues to disrupt our way of life through the rise of new variants and outbreaks. The world has experienced several pandemic waves, subsequent lockdowns, and a variety of different policies per country and sometimes even per region. The regular discovery of new variants made us realize that we need to transition from infrequent, symptomatic patient testing, towards a more frequent testing approach, called monitoring. The Omicron variant shows us that the current system is under strain, as vaccines and updates to these vaccines become a pressing requirement to keep pace with new variants popping up. It is becoming clearer by the day that the way out of this pandemic demands us to take an even more pro-active role in testing to prevent the spread of the virus.
In diagnostics, scientists have long been aware that we need to move from incidental and individual diagnostic testing to regular interval testing for monitoring on a much larger scale. Monitoring will be instrumental in keeping our society open. People will be regularly tested (twice per week). This will increase the chance of early detection of a new case of infection before that person becomes infectious and participates in spreading the virus. Being able to then isolate the individual temporarily, will stop the spread of COVID-19 in its tracks or at least significantly slow it down.
At MolGen we are highly motivated to enable this for the world, but it will be instrumental to motivate people to see the need to get tested twice a week. For this to work we will have to lift the barriers to mass PCR-testing and make collecting a sample non-invasive and the costs to governments, employers and individuals far more affordable.
How to lift barriers and make mass PCR-testing available and accessible
So how will we take away the public’s reluctance to get tested? We will have to take away the main barriers:
- Replace invasive nasal and throat swabs with a non-invasive sample collection method, using a Saliva Collection Kit.
- Make sample collection available at home, work, and any other location, instead of having to schedule an appointment at a local sampling location.
- Enabling the individual to easily link the sample to the person, without the fear of leaking or abuse of personal data, using our anonymous mobile app.
- Decrease the current costs of testing. By industrializing PCR-testing, the costs of a PCR-test will go down, making it suitable for mass monitoring purposes.
MolGen’s MegaPrep is an innovative solution that contains solutions to all these 4 barriers. By working with leading laboratories across the Netherlands, Europe, and the world, the MegaPrep workflow uses the same principles as any other COVID-19 extraction and PCR detection workflow but is implemented on an industrial scale. A combination of smart innovations in automation and massive parallel PCR can handle much larger amounts of samples in a shorter timeframe at lower cost.
Our Saliva Collection Kit mixes saliva with a stabilization lysis transport buffer. This tube can be either collected in a dropbox or can be sent in a safety bag to a lab where the PCR testing is performed. The barcode on the tube combined with video recording of the sample collection in the MegaPrep will allow for non-invasive, safe & anonymous sample collection.
MegaPrep is handling up to a million samples a day
By making non-invasive testing available every 3-4 days, we are far more likely to detect an infected person before they become infectious. This means testing twice a week, every week. By accommodating this at the workplace, at the gym and in our schools, we can create what I call green bubbles. Monitoring using large scale PCR-testing is the safest way to create and maintain these safe bubbles.
I’m excited about all the possibilities created by MegaPrep. Non-invasive, cost-effective, and sensitive testing. MegaPrep will forge the future of testing and will be a key to unlocking our world.
- Learn more about MolGen at molgen.com







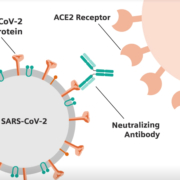
 ZTA Biotech, a Budapest-based biotech startup has announced the breakthrough development of a COVID-19 antibody (IgG) test using the ELISA protocol. This new detection method represents a great step forward in determining if patients have had COVID-19 and if they might still have immunity to the disease. Early results have proven 100% in specificity after testing 280 samples, and 100% sensitivity by testing 260 samples of recovered and symptomatic patients.
ZTA Biotech, a Budapest-based biotech startup has announced the breakthrough development of a COVID-19 antibody (IgG) test using the ELISA protocol. This new detection method represents a great step forward in determining if patients have had COVID-19 and if they might still have immunity to the disease. Early results have proven 100% in specificity after testing 280 samples, and 100% sensitivity by testing 260 samples of recovered and symptomatic patients.