Rapid and reliable detection of medulloblastoma-associated DNA methylation patterns: MS-MIMIC
Disease-associated variations in DNA methylation profiles hold significant potential for diagnostic and research applications. Unfortunately, scant and degraded samples often limit the analyses which can be performed. To overcome these issues, we developed Mass Spectrometry Minimal Methylation Classifier (MS-MIMIC) to identify then reliably analyse disease-specific DNA methylation profiles. The technique has now been validated in a cohort of pediatric medulloblastoma cases.
by Dr Ben Chaffey, Dr Debbie Hicks, Dr Edward Schwalbe and Prof. Steve Clifford
Background
Altered DNA methylation patterns have emerged as valuable biomarkers of disease pathogenesis, showing clear potential in diagnostics, sub-classification and prediction of therapeutic response/ disease course [1–7]. However, clinical assessment of these altered patterns can be problematic, with sample materials often being degraded/scant, such as formalin-fixed paraffin-embedded (FFPE) tissue and core biopsies, and certain platforms, such as DNA methylation microarrays, having a requirement for batched assessments of relatively large numbers of samples. This compromises generation of data from real-world samples within clinically meaningful timeframes, hampering translation of research findings into routine practice.
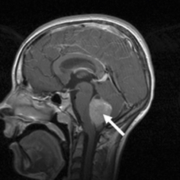
In this project, we focused on developing a new DNA methylation state assay to provide molecular subgrouping of cases of medulloblastoma (Fig. 1), the most frequently occurring malignant brain tumour in children. This disease has an approximate incidence of 1.5 cases per million, rising to 6 per million in children aged 1–9 years. It also occurs in adults, although in this group it is around ten times less common [8].
Although rates of survival to 5 years and beyond following diagnosis are around 65–70%, medulloblastoma still causes around 10% of all childhood cancer deaths. Initial treatment generally consists of complete or near complete surgical resection followed by adjuvant treatment with both post-operative radiotherapy and chemotherapy. Despite the fact that survival rates have improved over past decades, the delivery of individualized therapies based on patient-specific disease-risk profiles remains a major goal; intensified treatment for poor-risk disease, while reducing therapy for favourable-risk cases, with the overall aim of maximizing survival while minimizing late effects [9].
Medulloblastomas can be placed into one of four distinct subgroups, which are defined by specific methylomic, transcriptomic and genomic features. These are WNT, SHH, Group 3 and Group 4 [10]. Each group displays characteristic clinical and pathological features, drug targets and outcomes, and contributes significantly to the 2016 World Health Organization (WHO) classification of brain tumours [11]. Molecular subgrouping is, therefore, an important step in determining the most appropriate course of treatment and follow-up for individual patients [12].
Mass spectrometry minimal methylation classifier (MS-MIMIC) assay
The assay we have developed and validated, MS-MIMIC, is a novel polymerase chain reaction (PCR)-based assay for the multiplexed assessment of multiple signature CpG loci. We first identified a DNA methylation signature of 17 CpG loci using genome-scale Illumina 450k DNA methylation microarray data from 220 medulloblastoma cases. The 50 most discriminatory CpG loci for each molecular subgroup (200 loci in total) were considered as candidates for inclusion in the signature set. These were triaged using a 10-fold cross validated classification fusion algorithm, followed by a reiterative primer design process where amenability to primer design and multiplex bisulfite PCR was assessed in silico before finally undergoing in vitro PCR validation.
Candidate signature CpG loci were then analysed by a specific custom iPLEX assay [13] (Agena Bioscience). In this method (displayed schematically in Fig. 2), methylation-dependent SNPs representative of CpG methylation status are induced by initial treatment of DNA with sodium bisulfite [14] followed by multiplexed target-region amplification PCR, then single base extension and termination of target-specific probe oligonucleotides. The products of this reaction are analysed using MALDI-ToF (matrix-assisted laser desorption and ionisation – time of flight) mass spectrometry (MassARRAY System, Agena Bioscience). Each potential CpG locus variant yields a product with a unique and characteristic mass, enabling their rapid and unambiguous identification. MALDI-ToF analysis of single base variants is widely used to provide clinical DNA diagnostics in related genotyping applications [15], and is the key technical innovation which enables the robust assessment of medulloblastoma molecular subgroup, especially for samples which are refractory to analysis using conventional DNA methylation-array based methods.
Using these techniques, we generated an optimal, multiply-redundant 17-CpG locus signature and a robust assay for its detection.
A Support Vector Machine (SVM) classifier for the signature was then developed, using the existing 450k DNA methylation array data as a training set. SVM is a supervised machine learning technique commonly used in multiple areas of data analysis, including analysis of microarray data [16], making it well-suited to this application. Crucially, it returns a probability of group membership, enabling the assessment of confidence of subgroup assignment.
Next, we assessed MS-MIMIC performance against Illumina 450k methylation microarrays using an independent validation cohort of 106 medulloblastoma DNA samples which contained examples of all four medulloblastoma subgroups. These samples were also derived from tissue which reflected different clinical fixation methods commonly in use; fresh-frozen biopsies (n=40), FFPE tumour section (n=39), or FFPE-derived nuclear preparations [17] (n=27) produced by cytospin, a pre-analytical method that uses centrifugation to create a monolayer of cells for analysis on a slide from a low-concentration cellular suspension sample [18]. In this validation cohort, MS-MIMIC faithfully recapitulated DNA methylation array molecular subgroup assignments.
Quality control measures for CpG locus-specific assay failure were established; up to six failed CpG loci per sample were tolerated within the multiply-redundant signature/classifier, without impacting performance. Forty-three out of 106 validation cohort samples were affected by at least one locus failure, reflecting the damaged nature of DNA generally obtained from some of these samples. Five out of 106 samples had more than seven failed CpGs and were deemed not classifiable (NC). Molecular subgroup classifications were then compared, with MS-MIMIC classifications showed complete concordance with the reference subgroup, as determined by DNA methylation array [10]. Furthermore, CpG-level methylation estimates (β-values) were equivalent between methods (R2 = 0.79). As anticipated, fresh-frozen derivatives performed best (n=39/40; 98% successfully subgrouped), with 91% success (n=56/61) using FFPE-derived DNA from tumour sections and cytospin preparations (Fig. 3a–c).
Application of MS-MIMIC to the HIT-SIOP-PNET4 clinical trials cohort
Following successful assay development and validation, we next wished to test MS-MIMIC methylation signature detection in limited, poor quality, archival, clinical biopsies. Analysis of remnant material from the HIT-SIOP-PNET4 cohort [17] offered the first opportunity to determine the potential utility of molecular subgroup status to predict disease outcome in a clinical trial of risk-factor negative, ‘standard risk’ (SR) medulloblastoma. Only FFPE sections (n=42/153 available tumour samples) and cytospin nuclear preparations (approximately 30 000 nuclei isolated and centrifuged onto microscope slides; n=111/153) remained from this study archive and all DNA preparations fell below quality and quantity thresholds (>200 ng double-stranded DNA (dsDNA)) required for methylation profiling using conventional research methods (Illumina 450k and MethylationEpic arrays [16]). Using MS-MIMIC, 70% (107/153) of samples were successfully subgrouped, and subgroup assignments and β-value estimations were consistent across duplicate determinations. Assay performance was equivalent across the input DNA range (<2 ng (limit of detection) to 100 ng dsDNA (41.4 ng median DNA input).
Reasons for assay failure included unsuccessful bisulfite conversion/PCR (6%; 9/153), and inability to classify due to assay QC failure (24%; 37/153). These findings from HIT-SIOP-PNET4 reveal important subgroup-dependent molecular pathology in SR medulloblastoma. Group 4 was most common (n=62; 58%), with approximately equivalent numbers of WNT (18/170; 16%), SHH (17/107; 16%) and Group 3 (10/107; 9%) tumours observed. The majority (11/13) of events (defined as disease recurrence or progression following treatment) affected Group 4 patients [82% 5-year progression-free survival (PFS)], with >95% PFS in non-Group 4 patients. Subgroup assignment will thus be essential to inform future clinical and research studies in SR medulloblastoma.
Discussion
Detection of disease-specific variations in DNA methylation patterns has great potential for both supporting biomedical research and improving the quality of care that is delivered to patients. MS MIMIC has so far only been applied to medulloblastoma but this approach has clear potential for use in other cancers [7] and in other diverse settings, for example smoking [1], obesity [2], human fetal alcohol spectrum disorder [3] and aging [4].
Key resources which must be available for development of an MS-MIMIC assay for a given condition are a suitable collection of data concerning disease-state specific methylation patterns obtained using an array system such as those mentioned above, samples with which to perform assay validation, bioinformatics knowledge and support to create, optimize and operate the disease-specific SVM classifier system, plus access to a MassARRAY System for analysis. MS-MIMIC is discussed in greater detail in Schwalbe et al., 2017 [19].
References
1. Besingi W, Johansson A. Smoke-related DNA methylation changes in the etiology of human disease. Hum Mol Genet 2014; 23: 2290–2297.
2. Wahl S, Drong A, Lehne B, Loh M, Scott WR, Kunze S, Tsai PC, Ried JS, Zhang W et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 2017; 541: 81–86.
3. Portales-Casamar E, Lussier AA, Jones MJ, MacIsaac JL, Edgar RD, Mah SM, Barhdadi A, Provost S, Lemieux-Perreault LP et al. DNA methylation signature of human fetal alcohol spectrum disorder. Epigenetics Chromatin 2016; 9: 1–20.
4. Ong ML, Holbrook JD. Novel region discovery method for Infinium 450 K DNA methylation data reveals changes associated with aging in muscle and neuronal pathways. Aging Cell 2014; 13: 142–155.
5. Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, Pace TW, Rex-Haffner M, Loeschner A, Gonik M et al. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc Natl Acad Sci USA 2013; 110: 8302–8307.
6. Bacalini MG, Gentilini D, Boattini A, Giampieri E, Pirazzini C, Giuliani C, Fontanesi E, Scurti M, Remondini D et al. Identification of a DNA methylation signature in blood cells from persons with Down Syndrome. Aging 2015; 7: 82–96.
7. Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, Pfaff E, Tönjes M, Sill M et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 2012; 22: 425–437.
8. Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci 2012; 19: 1541–1544.
9. Pizer BL, Clifford SC. The potential impact of tumour biology on improved clinical practice for medulloblastoma: progress towards biologically driven clinical trials. British Journal Of Neurosurgery 2009; 23: 364–375.
10. Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 2012; 123, 465–472.
11. Louis DN, Cavenee WK, Ohgaki H, Wiestler OD. WHO classification of tumours of the central nervous system, 4th edn. pp.184–200. IARC Press, 2016.
12. Schwalbe EC, Williamson D, Lindsey JC, Hamilton D, Ryan SL, Megahed H, Garami M, Hauser P, Dembowska-Baginska B et al. DNA methylation profiling of medulloblastoma allows robust subclassification and improved outcome prediction using formalin-fixed biopsies. Acta Neuropathol 2013; 125: 359–371.
13. Gabriel S, Ziaugra L, Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Current Protocols in Human Genetics, Chapter 2: Unit 2.12. Wiley & Sons 2009.
14. Wang RY, Gehrke CW, Ehrlich M. Comparison of bisulfite modification of 5-methyldeoxycytidine and deoxycytidine residues. Nucleic Acids Res 1980; 8: 4777–4790.
15. Griffin TJ, Smith LM. Single-nucleotide polymorphism analysis by MALDI-ToF mass spectrometry. Trends Biotechnol 2000; 18: 77–84.
16. Hovestadt V, Remke M, Kool M, Pietsch T, Northcott PA, Fischer R, Cavalli FM, Ramaswamy V, Zapatka M et al. Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol 2013; 125: 913–916.
17. Clifford SC, Lannering B, Schwalbe EC, Hicks D, O’Toole K, Nicholson SL, Goschzik T, Zur Mühlen A, Figarella-Branger D et al. Biomarker-driven stratification of disease-risk in non-metastatic medulloblastoma: Results from the multicentre HIT-SIOP-PNET4 clinical trial. Oncotarget 2015; 6: 38827–38839.
18. Koh CM. Preparation of cells for microscopy using cytospin. Meth Enzymol 2013; 533: 235–240.
19. Schwalbe EC, Hicks D, Rafiee G, Bashton M, Gohlke H, Enshaei A, Potluri S, Matthiesen J, Mather M et al. Minimal methylation classifier (MIMIC): A novel method for derivation and rapid diagnostic detection of disease-associated DNA methylation signatures. Sci Rep 2017; 7: 13421.
The authors
Ben Chaffey1 PhD, Debbie Hicks2 PhD, Edward Schwalbe3 PhD, Steve Clifford2* PhD
1NewGene Ltd, International Centre for Life, Newcastle-upon-Tyne, UK
2Wolfson Childhood Cancer Research Centre, Northern Institute for Cancer Research, Newcastle University, Newcastle-upon-Tyne, UK
3Northumbria University, Newcastle-upon-Tyne, UK
*Corresponding author
E-mail: steve.clifford@ncl.ac.uk