A novel approach in the diagnostics of renal cell cancer: Image guided optical biopsy
Optical coherence tomography (OCT) has long been routinely used in ophthalmology, but recent studies in the field of renal cell carcinoma have demonstrated the ability of OCT to distinguish between renal malignancies and normal renal tissue. This suggests the possibility that, eventually, diagnosis by invasive biopsy could be replaced by non-invasive techniques.
by D. M. de Bruin, Dr P. Wagstaf, Dr K. Barwari, Prof. T. G. van leeuwen, Dr D. J. Faber, Prof. J. J. de la Rosette and Dr M. P. Laguna
The diagnosis of small renal masses
The diagnosis of small renal masses (SRMs) has seen a dramatic increase in presentation in recent decades. This change is mainly attributed to an increased use of abdominal imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI). However, the large imaging depth of such modalities is accompanied by a relatively low resolution of the obtained images, hindering conclusions at the level of histological composition. Recent studies have shown an inverse correlation between tumour size and malignancy, and up to 10 % of all extirpated (and thus deemed malignant) tumours appear to be benign on histopathological examination. This inverse relationship increases to 25% when small renal masses (SRM) (≤4 cm) are considered [1]. Therefore, pre-operative diagnosis of (small) renal tumours would be desirable. However, due to the high number of non-diagnostic biopsy results (up to 30 % in SRM), systematic use of pre-operative renal mass biopsies is still not recommended in the major guidelines [2–5].
Renal mass biopsy
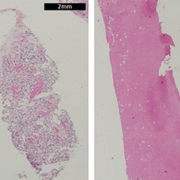
Most renal biopsies are performed percutaneously and are supported by image guidance using computed tomography (CT) or ultrasound. The biopsies are normally performed under local anesthesia in an outpatient setting. When a renal tumour is evaluated, a biopsy can deliver one of two results: diagnostic (benign or malignant) or non-diagnostic, the later including the presence of necrosis, fibrosis and normal renal parenchyma with absence of tumour cells [Figure 1]. When the biopsy is diagnostic, other characteristics such as histopathologic subtype and grade can also be assessed [4, 6, 7].
Conceptually a failed biopsy means that there is no tumour tissue available for assessment in the biopsy specimen, although other types of tissue might be present in the sample. The reason for a failed biopsy could be a technical failure of the puncture method (e.g. misfire or malfunctioning of the biopsy gun) or incorrect sampling caused by imperfect image guidance. Incorrect sampling is sometimes unavoidable due to the nature of renal tumours, which may contain necrotic and fibrotic tissue, or be mixed in nature with solid and cystic components. Also, the presence of normal renal tissue implies that the sampling is incorrect, as very few renal masses are composed of normal renal tissue. The presence of fibrotic, inflammatory, fatty or necrotic tissue in the specimen means that a differential diagnosis between malignant and benign tumour cannot be made. Besides the fact that histopathological analysis requires time, it is also subject to a certain degree of discordance among different pathologists [8].
A diagnostic imaging tool that allowed real-time visualization of micro-scale tissue architecture and subsequent differentiation of tissue type during the procedure would accelerate and simplify the overall diagnostic procedure.
Optical imaging
Optical diagnostic imaging comprises a novel group of imaging modalities that provide information by assessing differences between incident and detected light caused by the interaction of light with tissue. Scattering and absorption are tissue-specific optical properties and, by assessing these interactions,
diffeent tissue types can be distinguished.
Optical imaging has shown potential in several medical fields where they are employed routinely in various forms, ranging from pulse oximeters to fundus cameras, and experimental reports show promising results in the field of oncology [9].
Optical coherence tomography (OCT) is a technology developed in the early 1990s for ophthalmological applications [10] and is routinely used in that setting in current clinical practice. OCT is the optical equivalent of ultrasound, using light instead of sound to produce micrometer-scale resolution, cross-sectional images up to a depth of about 2 mm in renal tissue [Figure 2]. Resolutions up to 5 µm can be achieved, being 100–250 times higher than high-resolution ultrasound [11] and approaching that of microscopy. An image produced by OCT resembles the tissue structures observed in histology and can, therefore, be considered as an ‘optical biopsy’ [12] [Figure 2]. Moreover, data extracted from the original OCT images can be used for functional quantitative analysis after careful calibration of the OCT system. This finally results in a ‘functional optical biopsy’. The imaging depth is primarily limited by the scattering of light by cellular structures, hindering the return of reflections to the receiver. This scattering causes the light intensity to attenuate as it penetrates deeper into the tissue and this attenuation of OCT signal can be quantified by measuring the decay of signal intensity per unit depth. Using Lambert–Beer’s law and after careful calibration of the OCT system, a tissue specific attenuation coefficient (μOCT mm-1) can be derived [13–15]. Because malignant tissue displays an increased number, larger and more irregularly shaped nuclei with a higher refractive index and more active mitochondria, the μOCT is expected to be higher compared to normal and benign tissue [Figure 3].
In urology, the early research on OCT has been focused on tissue diagnosis predominantly in bladder and prostate cancer [12, 16] and, more recently, attention has turned to the field of renal cell carcinoma (RCC) and research is currently ongoing [17–20]. We were the first authors to publish data on the ability of OCT to differentiate renal malignancies from normal renal tissue using quantitative analysis. Subsequently, we performed an in vivo pilot study assessing the difference of the attenuation-coefficient of malignant renal tumours from normal renal parenchyma and benign tumours [18]. OCT-imaging took place using an in vivo OCT-probe during surgery, and a significant difference was found between the attenuation-coefficient of normal renal tissue and that of malignant tumours. Attenuation-coefficients of malignant and benign tumours did differ, although it is likely that the small sample size (3 benign tumours vs 11 malignant) is hindering a statistical significance, suggesting that a clear difference might be found in larger samples. Linehan et al. assessed qualitative differences of OCT images of different types of renal tumours showing that certain tumour subtypes do have different appearances on OCT-imaging; however, intriguingly, clinical distinction of tumours such as RCC from oncocytomas could not be demonstrated [19].
Future developments
Finally, anticipating the validation of results showing optical diagnostics being able to differentiate renal tissues, there is a potential role for the techniques in several clinical scenarios. Before going as far as replacing pathological examination as discussed earlier, the two techniques might be complementary with the real-time- and non-invasive nature of the optical techniques serving as guidance for correct needle placement in order to reduce the number of non-diagnostic biopsy results, as is already done in other malignancies, and the small in vivo probes necessary for such interventions are becoming commercially available. The technological configuration behind OCT allows for easy integration with diffuse reflectance spectroscopy (DRS) and Raman spectroscopy (RS). Moreover, the structural-imaging- and light-scattering based quantitative possibilities of OCT together with the quantitative light absorption sensitivity of DRS and the inelastic light scattering (and therefore biochemical) sensitivity of RS yields the full potential of a functional optical biopsy.
We would like to thank the Cure for Cancer Foundation (CFC) and the Technology Foundation (STW) for project funding. This work is part of the innovative Medical Imaging Technologies program (iMIT) of STW and the Novel Biopsy Methods program of CFC.
References
1. Tan H-J et al. Understanding the role of percutaneous biopsy in the management of patients with a small renal mass. Urology 2012; 79(2): 372–377.
2. Volpe A, Jewett MA. Current role, techniques and outcomes of percutaneous biopsy of renal tumors. Expert Rev Anticancer Ther 2009; 9(6): 773–783.
3. Motzer RJ et al. NCCN clinical practice guidelines in oncology: kidney cancer. J Natl Compr Canc Netw 2009; 7(6): 618–630.
4. Leveridge MJ et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol 2011; 60(3): 578–584.
5. Ljungberg B et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 2010; 58(3): 398–406.
6. Menogue SR et al. Percutaneous core biopsy of small renal mass lesions: a diagnostic tool to better stratify patients for surgical intervention. BJU Int 2012; doi: 10.1111/j.1464-410X.2012.11384.x.
7. Laguna MP et al. Biopsy of a renal mass: where are we now? Curr Opin Urol 2009; 19(5): 447–453.
8. Kümmerlin IP et al. Cytological punctures in the diagnosis of renal tumours: a study on accuracy and reproducibility. Eur Urol 2009; 55(1): 187–198.
9. Pierce MC, Javier DJ, Richards‐Kortum R. Optical contrast agents and imaging systems for detection and diagnosis of cancer. Int J Cancer 2008; 123(9): 1979–1990.
10. Huang D et al. Optical coherence tomography. Diss. Massachusetts Institute of Technology, Whitaker College of Health Sciences and Technology, 1993.
11. Fujimoto, JG et al. Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia 2000; 2(1–2): 9–25.
12. Crow P et al. Optical diagnostics in urology: current applications and future prospects. BJU Int 2003; 92(4): 400–407.
13. Faber DJ et al. Quantitative measurement of attenuation coefficients of weakly scattering media using optical coherence tomography. Optics Express 2004; 12(19): 4353–4365.
14. van Leeuwen TG, Faber DJ, Aalders MC. Measurement of the axial point spread function in scattering media using single-mode fiber-based optical coherence tomography. IEEE Journal of Selected Topics in Quantum Electronics 2003; 9(2): 227–233.
15. de Bruin DM et al. Optical phantoms of varying geometry based on thin building blocks with controlled optical properties. J Biomed Opt 2010; 15(2): 025001.
16. Cauberg EC et al. Quantitative measurement of attenuation coefficients of bladder biopsies using optical coherence tomography for grading urothelial carcinoma of the bladder. J Biomed Opt 2010; 15(6): 066013.
17. Barwari K et al. Advanced diagnostics in renal mass using optical coherence tomography: a preliminary report. J Endourol 2011; 25(2): 311–315.
18. Barwari K et al. Differentiation between normal renal tissue and renal tumours using functional optical coherence tomography: a phase I in vivo human study. BJU Int 2012; 110(8 Pt B):E415–20.
19. Linehan JA et al. Feasibility of optical coherence tomography imaging to characterize renal neoplasms: limitations in resolution and depth of penetration. BJU Int 2011; 108(11): 1820–1824.
20. Onozato ML et al. Optical coherence tomography of human kidney. J Urol 2010; 183(5): 2090–2094.
The authors
D. Martijn de Bruin1,2,* Msc; Peter G. Wagstaff1 MD; Kurdo Barwari1 PhD, MD; Ton G. van Leeuwen2 PhD; Dirk J. Faber2 PhD; Jean J. de la Rosette1 PhD, MD; M. Pilar Laguna1 PhD, MD.
1 Department of Urology, Academic Medical Center, Amsterdam, Meibergdreef 9, 1105 AZ, The Netherlands
2 Department of Engineering & Physics, Academic Medical Center, Amsterdam, Meibergdreef 9, 1105 AZ, The Netherlands
*Corresponding author
E-mail: d.m.debruin@amc.uva.nl