Anti-DFS70 antibodies: detection and significance
by Professor M. Herold
Systemic autoimmune rheumatic diseases (SARD) are rare and difficult to diagnose. Antinuclear antibodies (ANAs) are one of the most important serological features in the diagnostic work-up of patients with SARD. However, up to 30% of healthy individuals tested are also positive for ANAs and in about 50% of these cases ANA positivity is based on anti-DFS70 antibodies. Anti-DFS70 antibodies show a typical dense fine speckled (DFS) pattern on HEp-2 cells and can be identified by specific immunoassays. The presence of isolated anti-DFS70 antibodies seems to be a serological sign that an individual does not have SARD.
Systemic autoimmune rheumatic diseases and antinuclear antibodies
Antinuclear antibodies (ANAs), directed against intracellular antigens, are found in patients with different autoimmune diseases and are an important part of the diagnostic procedure. ANA positivity in serum is part of several classification criteria of systemic autoimmune rheumatic diseases (SARD) such as systemic lupus erythematosus (SLE), Sjögren’s syndrome and scleroderma. The indirect immunofluorescence test on HEp-2 cells is the recommended ‘gold standard’ test for the detection of ANAs and enables scanning for antibodies to a large number of putative autoantigens present in the nucleus and other cell compartments. Many of these antibodies seen on HEp-2 cells have no known connection to a particular pathology or disease. Specific autoantibodies show typical fluorescence patterns. In a follow-up these antibodies are identified with different analytical methods using specific antigens. In contrast to the indirect immunofluorescence test these immunoassays can only detect a limited number of autoantibodies with well known clinical or diagnostic relevance. It is not surprising that ANAs detected by indirect immunofluorescence can also be found in healthy individuals. In general the serum level of ANAs is lower in persons without SARD than in patients with autoimmune diseases and cut-off levels are defined to discriminate between patients with SARD from those without SARD. However, ANAs might be the first sign that a patient with no clinical symptoms has early SARD – pre-dating the onset of an autoimmune disease as it was described for rheumatoid arthritis, myositis and SLE. Nevertheless, sera from 20–30% of healthy people screened for ANAs by indirect immunofluorescence using HEp-2 cells tests positive depending on age of the individual and the screening titre. About half of these ANA-positive sera of healthy individuals show antibodies directed against the dense fine speckles 70 (DFS70) antigen. The primary target was identified as the lens epithelium derived growth factor (LEDGF), which is also known to be the DNA binding transcription coactivator p75. This protein has a number of physiological functions. Among others, DFS70/LEDGF promotes cell survival and enhances resistance to cellular stress. It is a cofactor for HIV replication through an interaction with HIV-1 integrase activity. DFS70/LEDGF is also highly expressed in prostate tumour tissue.
Anti-DFS70 antibodies
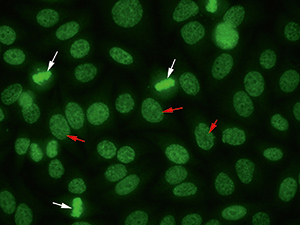
Anti-DFS70 antibodies can be recognized within the routine screening test for ANA by indirect immunofluorescence on HEp-2 cells showing a uniformly distributed fine speckled nuclear staining in interphase cells and a positive fine speckled fluorescence of similar appearance in the chromosomes of mitotic cells (Fig. 1). As a 70 kD protein was recognized as the antigen, the corresponding antibodies were named antibodies against dense fine speckles 70 (anti-DFS70).
Anti-DFS70 antibodies have been described in patients with different chronic inflammatory diseases but an association to a specific disease could not be established. Anti-DFS70 antibodies were initially identified among ANAs in the serum of a patient with interstitial cystitis. Comparisons of the antibody’s prevalence in various diseases reveal the highest prevalence in Japanese patients with atopic dermatitis (30%). In the United States a remarkably high prevalence was found in patients with bronchial asthma (16%), as well as in other allergy disorders and in patients with interstitial cystitis (9%). A high number of patients with alopecia areata also seem to have anti-DFS70 antibodies. In a Japanese investigation sera from 59 individuals out of 111 (53%) patients with alopecia areata were positive for ANAs and out of them close to 20% (22/111) were positive for anti-DFS70 antibodies. Also in patients with prostate cancer anti-DFS70 antibodies were detected in an increased number (18%). It is thought that LEDFG/p75 cleavage fragments generated during prostate tumour cell death might trigger autoantibodies under certain inflammatory conditions.
The presence of autoantibodies is usually taken to be a sign of autoimmune reaction or autoimmune disease. However, anti-DFS70 antibodies do not seem to be associated with the presence of SARD or any other pathological autoimmune reaction.
Therefore, anti-DFS70 antibodies have been proposed as useful biomarkers for the exclusion of SARD. This suggestion is mainly based on observations that anti-DFS70 antibodies are more often found in individuals with no evidence of active SARD than in patients with these diseases. Interestingly, anti-DFS70 titres in healthy individuals are usually high (with titres >1:640) and other autoantibodies are not found. In a clinical follow-up at four years, anti-DFS70 antibody-positive healthy individuals did not develop SARD. In contrast, patients with SARD and anti-DFS70 antibodies (less than 3%) usually also have positive results for other disease specific antibodies.
Differences in age and sex distribution of anti-DFS70 antibodies
It is well known that, in general, the prevalence of ANAs increases with age. The reason is unclear. One idea among several is that the increased occurrence of autoantibodies in elderly people reflects normal immunosenescence within the process of aging. Surprisingly, ANAs against dense fine speckles (anti-DFS70 antibodies) are more often found in younger people and the number of anti-DFS70 antibody-positive individuals decreases with increasing age. The reason for a lower incidence of anti-DFS70 antibodies in elderly people is unknown. One possibility is that our immune systems have been stimulated to produce anti-DFS70 antibodies only recently (with the last few decades) in response to unknown influences.
The sex distribution of anti-DFS70 antibodies also shows differences with a greater preponderance in females.
Prevention of misleading results
A high titre of anti-DFS70 antibodies in an ANA-positive sample (causing a false-positive effect) might mislead the clinical judgement of physicians if they are not aware that anti-DFS70 antibodies seem to exclude an autoimmune disease. It is supposed that classification criteria for SARD including ANA positivity should be revised to reflect this. Sera positive for ANAs should be tested to check that they are not directed against DFS70 before including the result in a diagnosis of SARDs. DFS70 positive samples may be found by indirect immunofluorescence on HEp-2 cells if technicians are trained to recognize the specific DFS70 pattern. If follow-up of ANA-positive samples with DFS70 pattern reveals no positive result in any well known disease specific subtype other than anti-DFS70 antibodies the latter may be presumed. The confirmation is given by specific tests using recombinant DFS70 to detect the specific IgG antibodies. Different commercial assays are available including a fully automated chemiluminescence immunoassay (INOVA Diagnostics Inc.), a semi-quantitative enzyme-linked immunosorbent assay (MBL Medical & Biological Laboratories Co.) and an immunoblot for the detection of autoantibodies against DFS70 and other ANA-subtypes (D-Tek s.a. and IMMCO Diagnostics).
Summary
Anti-DFS70 autoantibodies have been known of for several years but general interest in clinical laboratories has increased since specific immunoassays for the detection of anti-DFS70 antibodies became commercially available. About one out of five serum samples from healthy individuals who are tested for ANAs yields a positive result, which is in most cases caused by anti-DFS70 antibodies. Confirmation by specific immunoassay is useful to avoid further diagnostic procedures as anti-DFS70 antibodies are not associated with autoimmune diseases.
Bibliography
1. Avery TY, et al. Anti-nuclear antibodies in daily clinical practice: prevalence in primary, secondary, and tertiary care. J Immunol Res. 2014; 2014: 401739.
2. Bizzaro N, et al. Antibodies to the lens and cornea in anti-DFS70-positive subjects. Ann N Y Acad Sci. 2007; 1107: 174–183.
3. Daniels T, et al. Antinuclear autoantibodies in prostate cancer: immunity to LEDGF/p75, a survival protein highly expressed in prostate tumors and cleaved during apoptosis. Prostate 2005; 62: 14–26.
4. Ganapathy V, Casiano CA. Autoimmunity to the nuclear autoantigen DFS70 (LEDGF): what exactly are the autoantibodies trying to tell us? Arthritis Rheum. 2004; 50: 684–688.
5. Mahler M, Fritzler MJ. The clinical significance of the dense fine speckled immunofluorescence pattern on HEp-2 cells for the diagnosis of systemic autoimmune diseases. Clin Dev Immunol. 2012; 2012: 494356.
6. Mahler M, et al. Importance of the dense fine speckled pattern on HEp-2 cells and anti-DFS70 antibodies for the diagnosis of systemic autoimmune diseases. Autoimmun Rev. 2012; 11: 642–645.
7. Mahler M, et al. Anti-DFS70/LEDGF antibodies are more prevalent in healthy individuals compared to patients with systemic autoimmune rheumatic diseases. J Rheumatol. 2012; 39: 2104–2110.
8. Mariz HA, et al. Pattern on the antinuclear antibody-HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheum. 2011; 63: 191–200.
9. Miyara M, et al. Clinical phenotypes of patients with anti-DFS70/LEDGF antibodies in a routine ANA referral cohort. Clin Dev Immunol. 2013; 2013: 703759.
10. Okamoto M, et al. Autoantibodies to DFS70/LEDGF are increased in alopecia areata patients. J Autoimmun. 2004; 23: 257–266.
11. Watanabe A, et al. Arthritis Rheum. 2004; 50: 892–900.
The author
Manfred Herold MD, PhD
Innsbruck Medical University, Dept of Internal Medicine VI, 6020 Innsbruck, Austria
E-mail: manfred.herold@i-med.ac.at