Autoimmune thyroid diseases: diagnostic and predictive role of thyroid autoantibodies
Autoimmune thyroid diseases, comprised by Hashimoto thyroiditis, Graves disease, and their variants, are characterised histologically by lymphocytic infiltration of the thyroid gland, biochemically by the presence of well-defined autoantibodies, and clinically by the impairment of thyroid function. The aetiology and mechanisms of the autoimmune damage depend upon genes and environmental factors such as iodine intake and smoking.Three major autoantigens have been identified for autoimmune thyroid disease: thyroglobulin, thyroperoxidase and thyrotropin receptor. Antibodies against these autoantigens are now part of the clinical toolbox. They are not only used to confirm a diagnostic suspicion, but also to predict recurrence of future onset of thyroid autoimmunity.
by Dr Alessandra De Remigis and Dr Patrizio Caturegli
Background on autoimmune thyroid diseases
Autoimmune thyroid diseases (ATDs) comprise two major conditions: Hashimoto thyroiditis with goitre and euthyroidism or hypothyroidism, and Graves disease with goitre, hyperthyroidism and often ophthalmopathy. Hashimoto thyroiditis is named after Dr Hakaru Hashimoto who described in 1912 the thyroid pathological features of four women who had undergone thyroidecomty because of compressive symptoms [1]. Graves disease is named after Dr Robert Graves who reported three patients with hyperthyroid goitre and ocular involvement (Graves ophthalmopathy) [2]. Both conditions are characterised pathologically by infiltration of the thyroid gland with autoreactive T and B lymphocytes and biochemically by the production of thyroid autoantibodies and abnormalities in thyroid function. It is not uncommon to observe transition from one clinical picture to another within the same patient over time, suggesting the existence of common immunological mechanisms [3]. Numerous clinical variants are described for each ATD. For Hashimoto thyroiditis, in addition to the classical goitrous form, fibrous, juvenile, thyrotoxic, post-partum, and IgG4-related forms are described. For Graves disease, beside the classical goitrous form with hyperthyroidism, there is the variant with hyperthyroidism and ophthalmopathy, the one with just ophthalmopathy (called euthyroid Graves disease), and the one with ophthalmopathy and localised myxoedema. Similar to many other autoimmune diseases, ATDs can occur in isolation or associated with other autoimmune diseases, often affecting other endocrine glands. In some patients this association is clinically recognisable and defined as polyglandular autoimmune syndrome.
ATDs are the most common autoimmune diseases with a population prevalence around 2 % in women and 0.2% in men. These estimates are considered to be 10 times higher if ‘subclinical disease’ is taken into consideration [4].
The pathogenesis of ATDs remains to be elucidated but it is believed to rely on the interaction between endogenous genetic factors and exogenous environmental factors. Genes that confer susceptibility to ATDs have been investigated since the 1970s via candidate gene analysis, linkage analysis and genome-wide association studies. Only a handful of genes have been identified and confirmed to increase the risk of ATDs development, but each gene contributes only a very small effect, with odds ratios typically below 3. These genes include the class II region of the major histocompatibility complex, CTLA-4, PTPN22, CD40, CD25, FCRL3, thyroglobulin, and the TSH receptor [5]. Another endogenous factor that has been studied extensively in ATD is pregnancy [6],which is known to ameliorate disease severity. The two major environmental factors implicated in ATDs initiation and progression are iodine and smoking. High iodine intake triggers lymphocytic infiltration of the thyroid in genetically susceptible animals (BB/W rats and NOD.H-2h24 mice); and is associated with increased prevalence and incidence of autoimmune thyroiditis and overt hypothyroidism in humans [7]. Iodine supplementation should thus be kept within the WHO recommended range to prevent from one side iodine deficiency and from the other side autoimmune thyroiditis [8]. Smoking does not have a univocal effect on AITD. It increases the risk of developing Graves disease and aggravates Graves ophtalmopathy. Smoking cessation is associated wth a better response of Graves ophthalmopathy to immunosuppressive treatment [9]. However, smoking seems to have a beneficial effect on Hashimoto thyroiditis and decreases the levels of thyroid autoantibodies [10].
Significant progress has been accomplished on the identification and characterisation of the thyroid autoantigens that are targeted by the immune system in patients with ATDs, so that antibodies against thyroglobulin, thyroperoxidase and the TSH receptor are now well-established tools in the clinical arena.
Thyroid antigens and antibodies
Thyroglobulin
Thyroglobulin is a large glycoprotein made of two identical 330-kDa subunits composed of 2,768 amino acids. Each subunit contains 66 tyrosines that when iodinated and processed make up the thyroid hormones (T4 and T3). Thyroglobulin contains numerous immunodominant epitopes for both T and B lymphocytes. Some epitopes are located in the iodine-rich hormonogenic regions and are affected by the iodine content.
Thyroglobulin antibodies (TgAb) recognise predominantly conformational epitopes, tend to favour the IgG2 subclass and do not fix complement. TgAbs were originally detected by tanned red cell haemagglutination, then by quatitative RIAs or ELISAs, and more recently by automated chemiluminescent EIAs. The analytical sensitivity varies depending on the assay method used, and the cut-off value for positivity is typically set at 100 WHO units/mL.
TgAbs are a marker of underlying thyroid autoimmunity but can also be found in non-autoimmune thyroid diseases as well as in healthy controls [11]. Traditionally they are requested together with thyroperoxidase antibodies to corroborate a diagnostic suspicion of ATDs. Currently, however, the greatest utility of TgAb measurement is in the follow-up of patients with differentiated thyroid cancer. These patients undergo thyroidectomy, possibly combined with radioactive iodine administration, and are then followed by measuring thyroglobulin antigen in the serum. Since thyroglobulin is a thyroid-specific antigen, its serum levels should be undetectable after thyroid ablation in the absence of recurrence or metastasis. If the patient had autoimmune thyroiditis in addition to thyroid cancer, TgAbs can persist for years after thyroidectomy and interfere with the thyroglobulin antigen determination. In particular, they can cause falsely low or undetectable levels of thyroglobulin antigen, and therefore a false positive clinical assessement. This realisation has led to the introduction of reflex measurement of TgAbs any time thyroglobulin is measured in thyroid cancer patients. Numerous studies have attempted to distinguish the type of TgAb based on the specific epitopes they recognise. Latrofa and colleagues have recently reported a pattern of TgAb for patients with ATDs and another for patients with multinodular goitre and differentiated thyroid cancer [12]. TgAbs also seem to recognise distinct epitopes in healthy subjects and patients with clinically manifest disease [13], suggesting the potential clinical utility of TgAbs based on specific thyroglobulin epitopes rather than on the entire thyroglobulin molecule.
Thyroperoxidase
Thyroperoxidase is a large membrane-associated glycoprotein (933 amino acids with a molecular weight of approximately 105 kDa) expressed at the apical (follicular) side of the thyroid cell. It separates to the microvillar/microsomal fraction upon ultracentrifugation and for this reason was originally called M antigen. Thyroperoxidase antibodies (TPOAbs) are predominantly IgG, can fix complement and cause damage to the thyroid cell by cell-mediated cytotoxicity [14]. TPOAbs are considered more specific for autoimmune thyroiditis than TgAbs. They correlate directly with the number of autoreactive lymphocytes infiltrating the thyroid gland as well as with the degree of thyroid hypoechogenicity on thyroid ultrasound. Like TgAbs, TPOAbs were originally measured by semiquantitative methods, then by RIAs or ELISAs, and more recently by automated chemiluminescent EIAs. The analytical sensitivity, as for TgAbs, varies according the assay method used; and the cut-off for positivity is 100 WHO units/mL.
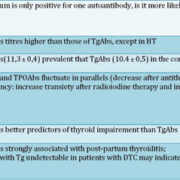
In the third National Health and Nutrition Examination Survey the presence of TPOAbs was strongly associated with TSH values greater than 4.5mUI/l and clinical hypothyroidism as well as with TSH values lower than 0.4 mUI/l and clinical hyperthyroidism [15]. These relationships were not observed for TgAbs, suggesting that their diagnostic and prognostic value is lower than that of TPOAbs [Table 1]. TPOAbs can thus be considered the best serological marker we currently have to establish or corroborate a diagnosis of autoimmune thyroiditis. They also have another unique clinical application in the prediction of post-partum thyroiditis. It has been shown that pregnant women who have TPOAbs at the beginning of pregnancy have a significantly greater risk to develop hypothyroidism in the first year after delivery, as well as permanent thyroid dysfunction in the long-term follow-up [16].
TSH Receptor autoantibodies
The thyrotropin receptor (TSHR) is a G protein-coupled glycoprotein composed of 764 amino acids with a molecular weight of approximately 87 kDa. It is composed of two subunits linked by disulphide bonds: a large extracellular A subunit at the N-terminus (residues 1-418) and a B subunit that spans the plasma membrane seven times and ends with a short cytoplasmic tail (residues 419-764). The region between residues 277 and 418 is called the hinge region, which is critical for defining the relationship among the various TSHR domains. After expression on the plasma membrane, the TSHR undergoes intramolecular cleavage so that a fragment of approximately 50 amino acids called C peptide is removed from the hinge region, leaving the A and B subunits linked by the disulphide bonds. After cleavage, some of the A subunits are shed from the cell surface. The TSHR is the master regulator of thyroid function, being involved in thyrocyte differentiation, proliferation and function [17].
Antibodies to the TSHR are found in Graves disease and are key mediators of the pathogenesis. Different categories of TSHR antibodies (TRAbs) have been identified: those with a stimulatory effect on the thyroid gland responsible for hyperthyroidism, those with an inhibitory effect on the receptor responsible for hypothyroidism, and those with neutral activity. TRAbs can be measured by immunoassays, which determine the presence and titre of the antibody but not the activity, and by bioassays. Immunoassays (the most commonly used) use a monoclonal antibody bound to a solid phase that recognises the native human TSHR produced by recombinant DNA technology in mammalian cells. Then bovine TSH labelled with biotin and the patient serum are co-incubated to compete for binding to the immobilised TSHR: the lower the signal the higher the titre of TRAbs in the patient’s serum. Sometimes it is important to establish not only whether TRAb are present but also their biological activity, usually to rule out blocking antibodies. Bioassays use cultured mammalian cells stably transfected with TSHR and then measure the increase (stimulating antibodies) or the decrease (blocking antibodies) in cAMP production, following the addition of the patient’s serum [Figure 1].
TRAbs are used in four main clinical settings: 1) to predict relapse and clinical course of Graves condition. For example, Graves disease patients with high TRAbs six months after diagnosis and medical treatment are more susceptible to relapse [18]; in addition, by combining TPOAbs and TRAbs measurements, the predictive power increases especially in those patients with moderately elevated TRAbs (6-10 IU/l) [19]; 2) to diagnose an autoimmune pathogenesis in patients with isolated Graves ophthalmopathy; 3) to distinguish in the post-partum period a thyrotoxicosis due to destructive thyroiditis from the hyperthyroidism due to Graves disease; and 4) to forecast the development of neonatal Graves disease in infants born to mothers with Graves disease.
Predictive role of thyroid autoantibodies in the natural history of ATD
In recent years there has been a resurgent interest in autoantibodies, for long considered just a mere disease biomarker rather than an important pathogenic player. Longitudinal studies of patients with autoimmune diseases have shown that autoantibodies precede the clinical diagnosis by several years, establishing the field of predictive antibodies. For ATDs, we have recently carried out a study in female US soldiers and shown that TPOAbs and TgAbs precede a clinical diagnosis of ATD by at least seven years in a significant percentage of the subjects [Figure 2], suggesting that when detected in healthy subjects, autoantibodies should not be overlooked because they can predict the onset of future clinically evident dysfunctions [20].
References
1. Hashimoto H. Archiv für Klinische Chirurgie 1912: 219-248.
2. RJ G. Newly observed affection of the thyroid gland in females (clinical lectures). Lond Med Surg J 1835.
3. Tamai H et al. J Clin Endocrinol Metab 1989; 69(1): 49-53
4. Weetman AP. Horm Res 1997; 48 Suppl 4: 51-54
5. Simmonds MJ, Gough SG. Clin Exp Immunol 2004; 136(1): 1-10
6. Landek-Salgado MA et al. Autoimmun Rev 2010; 9(3): 153-157
7. Teng W et al. N Engl J Med 2006; 354(26): 2783-2793
8. Sundick RS, Bagchi N, Brown TR. Autoimmunity 1992; 13(1): 61-68
9. Vestergaard P et al. Thyroid 2002; 12(1): 69-75
10. Belin RM et al. J Clin Endocrinol Metab 2004; 89(12): 6077-6086
11. Spencer CA et al. J Clin Endocrinol Metab 1998; 83(4): 1121-1127
12. Latrofa F et al. J Clin Endocrinol Metab 2008; 93(2): 591-596
13. Prentice L et al. J Clin Endocrinol Metab 1995; 80(3): 977-986
14. McLachlan SM, Rapoport B. Thyroid 2004; 14(7): 510-520
15. Hollowell JG et al. J Clin Endocrinol Metab 2002; 87(2): 489-499
16. Premawardhana LD et al. Thyroid 2004; 14(8): 610-615
17. Ludgate ME, Vassart G. Baillieres Clin Endocrinol Metab 1995; 9(1): 95-113
18. Schott M et al. Horm Metab Res 2005; 37(12): 741-744
19. Schott M et al. Horm Metab Res 2007; 39(1): 56-61
20. Hutfless S et al. J Clin Endocrinol Metab 2011; 96(9): E1466-71
The authors
Dr Alessandra De Remigis and Dr Patrizio Caturegli
Johns Hopkins University
Department of Pathology
Baltimore, MD, USA
e-mail: pcat@jhmi.edu