Biomarker diversity in lupus: challenges and opportunities
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease associated with diverse clinical manifestations. Accurate diagnosis, prediction of disease activity, organ involvement and management remains problematic owing to a lack of reliable biomarkers. This article reviews traditional and a few promising candidate biomarkers in SLE with specific clinical implications.
by Dr Anne E. Tebo
Background
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by heterogeneity in disease manifestations as well as diversity in immunologic and therapeutic responses. Despite longstanding research efforts, precise diagnosis and prediction of response to treatment remain problematic owing to variable disease presentation and course as well as a lack of sensitive and specific biomarkers [1, 2]. Several factors contribute to the immune abnormality and clinical heterogeneity that occurs in SLE; these include genetic, epigenetic, environmental, and hormonal influences. The interplay between these elements drives the production of a variety of autoantibodies, complement products, inflammatory markers and other mediators identified as biomarkers to diagnose, monitor, stratify and/or predict disease risk, course or response to treatment [1–7]. These biomarkers – genetic, biologic, biochemical or molecular – may correlate with disease pathogenesis or specific clinical manifestations and can be evaluated qualitatively or quantitatively in laboratories. Notable amongst these are diverse autoantibodies and complement products which (in addition to clinical manifestations such as skin lesions, arthritis, renal disorder, hematologic changes, neurologic disorder amongst others) are traditionally considered hallmarks of disease [8, 9]. This review article highlights traditional and a few promising candidate serologic, cellular and urine biomarkers for diagnosing and predicting disease activity as well as renal involvement in SLE.
Biomarkers for the diagnosis of SLE
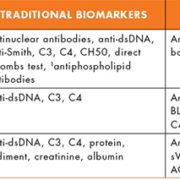
The updated American College of Rheumatology (ACR) revised criteria for the classification of SLE [8] is largely used in clinical practice to diagnose patients. In addition to specific clinical manifestations, the guidelines recommend testing for antinuclear antibodies (ANA), anti-double stranded deoxyribonucleic acid (anti-dsDNA), anti-Smith (anti-Sm) and antiphospholipid antibodies (lupus anticoagulant, IgG and IgM antibodies to cardiolipin (aCL) and beta2 glycoprotein I (anti-β2GPI). Recently, the Systemic Lupus Collaborating Clinics proposed the SLICC criteria for SLE in view of recent knowledge of the immunology of SLE [9]. Based on the SLICC rule for the classification of SLE, a patient must satisfy at least four criteria, including at least one clinical criterion and one immunologic criterion or must have biopsy-proven lupus nephritis (LN) in the presence of ANAs or anti-dsDNA antibodies. SLE is also associated with a variety of extractable nuclear antibodies such as anti-SSA, anti-SSB, and anti-snRNP as well as anti-ribosomal P, anti-histone, anti-nucleosome, anti-PCNA and anti-C1q autoantibodies [3, 5, 6]. The diagnostic characteristics of these autoantibodies (especially the anti-dsDNA antibodies) have been reported to be variable, which may be attributable the diversity of analytical methods, target antigens, patient demographics and SLE clinical subsets investigated [3, 5, 6, 11].
In addition to specific autoantibody tests, the proposed SLICC criteria recommend testing for complements C3, C4 and CH50 as well as using the direct Coombs assay. In the past several years, serum C3, C4 and CH50 levels have traditionally been used to diagnose and monitor disease activity in SLE patients [reviewed in 1, 2, 4]. However, in vitro activation may compromise interpretation of results and serum complement levels do not differentiate between consumption and production, which may be important for diagnosis. There is some evidence that cell-bound complement activation products (CB-CAPs) may facilitate SLE diagnosis [4, 12, 13]. These include complement C4-derived ligand deposited on erythrocytes (EC4d), platelets (PC4d), B lymphocytes (BC4d) and reticulocytes as detected by flow cytometry. Compared to disease controls, there is a relative increase of cell-bound C4d (CB-C4d) in SLE. However, the actual relevance of a single CB-C4d assay to the diagnosis of SLE is thought to be unlikely, although a panel of EC4d and BC4d assays is proposed to be predictive of SLE [12]. Further studies in diverse SLE clinical subsets and populations are required to determine the optimal CB-C4d panels for diagnostic evaluation.
Biomarkers for assessing disease activity
There are currently no consensus measures or biomarkers to reliably evaluate disease activity, predict flares and their differentiation from permanent damage in SLE patients. Disease activity indices such as the SLE disease activity index 2000 (SLEDAI-2K), British Isles Lupus Assessment Group (BILAG 2004), and the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI) are all complex and mostly used in academic centres and/or in clinical trials [reviewed in 14]. In addition to routine serologic markers for inflammation, anti-dsDNA antibodies, C3, and C4 have been traditionally used to assess disease activity and predict flares in SLE (Table 1). For patients with LN, urine analysis for protein, sediment, protein-creatinine ratio and albumin are used to evaluate activity, monitor treatment response and predict relapse. Several studies have examined the associations between traditional and non-traditional biomarkers to determine optimal panel of markers associated with disease activity or predicting severity [reviewed in 1–7]. The outcomes in these investigations have been inconsistent, probably due to variability in detection methods and heterogeneity in patient populations. These inconsistencies have hampered the adoption of an acceptable biomarker panel to evaluate and monitor disease activity.
A number of promising candidate biomarkers associated with disease activity in SLE have been identified and are reviewed in other publications [1, 2, 4–7]. These include biomarkers for serologic (autoantibodies, cytokines and cytokine receptors, markers of endothelial cell activation, and soluble cell surface molecules), cellular (cell-bound C4d, CD27high plasma cells and other lymphocyte subsets) and urine [neutrophil gelatinase-associated lipocalin (NGAL), sVCAM-1 (soluble vascular cell adhesion molecule 1), MCP-1 (monocyte chemotactic protein 1), and TWEAK (tumor necrosis factor-like weak inducer of apoptosis), immunoglobulin free light chain, von Willebrand factor, IL-6] analyses. Of the several autoantibodies described, anti-nucleosome and anti-C1q antibodies appear to significantly correlate with disease activity and/or predict flares [2, 4, 6]. Chromatin, the DNA-histone complex found in the nucleus is organized into a repeating series of nucleosomes. In SLE patients, anti-nucleosome antibodies are more likely to be detected in patients with LN and may serve as a useful biomarker in the diagnosis of active LN [reviewed in 2]. Anti-C1q IgG antibodies that target epitopes present within the collagen-like tail of C1q are also seen in varying prevalence in SLE patients. Increasing titres in anti-C1q antibodies have been suggested to predict renal flares [2, 4, 6]. Other serologic biomarkers such as cytokines (IFN-α/β, IFN-γ, IL-1, IL-6, IL-10, IL-12, IL-15, IL-17, IL-21, and TNF-α), IFN-inducible chemokines, a few cytokine receptors, complements, specific markers of endothelial cell activation, BLys and several others associated with SLE pathogenesis and disease activity have been described [1, 2, 4]. However, these have mostly been reported in research studies and are beyond the scope of this article.
Quite a number of studies have shown that elevated levels of complement activation cleavage products may reflect disease activity more accurately and are more likely than conventional measurements in the prediction disease flares. Of these, the best described is the Cd4 fragment, which is capable of binding several cell types. In one study, EC4d levels were observed to be higher in patients with ‘more active’ and ‘most active’ SLE compared with those with ‘less active’ disease [13]. EC4d measurements were also found to be associated with specific measures of disease activity even after adjusting for serum levels of C3, C4 and anti-dsDNA antibodies [13]. However, independent prospective investigations with appropriate controls are needed to validate these observations.
Biomarkers for renal involvement
Of the different clinical subsets of SLE, LN is one of the most common and associated with significant morbidity and mortality. In the US, approximately 35% of adults with SLE have clinical evidence of nephritis at the time of diagnosis, with an estimated 50–60% developing nephritis during the first 10 years of disease [reviewed in 15]. Among these patients, quite a few will progress to end-stage renal disease. Improved methods for detecting LN would allow earlier treatment preventing irreversible impairment of renal function and damage. In place of invasive, subjective and costly serial renal biopsies, tests such as creatinine clearance, levels of urine protein and sediment as well as serologic determinations of C3, C4, creatinine level and anti-dsDNA titres have for decades been used to follow the onset, course, and severity of LN. It is however, recognized that these analyses are inadequate as they are limited in responsiveness to change and therefore unsuitable for patient care [7, 15].
In an effort to reliably diagnose LN, several candidate biomarkers have identified [1, 2, 7]. Among autoantibodies, antichromatin/anti-nucleosome and anti-C1q antibodies have shown some promise as biomarkers of renal involvement as previously described in this article. Of the urine protein biomarkers NGAL, sVCAM-1, MCP-1 and TWEAK, amongst others, have received considerable attention (Table 1) [1, 2, 7]. Of these, NGAL has been much studied. It is a small protein expressed in the neutrophils and certain epithelial cells, including the renal tubules. Under normal physiologic conditions, NGAL expressions are low in urine and plasma, but quickly rise from basal concentrations in response to kidney injury to reach diagnostic thresholds within a very short period of time. This is in contrast to the routinely used kidney function tests such as creatinine, where increased concentrations may not be observed until 24 to 48 hours after injury and often lack sensitivity. Urine NGAL is, however, not specific for SLE and further studies are necessary to establish accurate reference ranges based on age, gender and ethnicity. Like NGAL, urine sVCAM-1, sICAM-1 (soluble intercellular CAM-1) and MCP-1 have been shown in human studies to be strongly correlated with LN activity and severity [reviewed in 7]. With the identification of these novel urinary biomarkers for diagnosing and differentiating active versus inactive LN, several studies have examined their relationship with histological features of LN. Brunner et al. 2012 examined a number of established markers (anti-dsDNA, serum C3, C4, creatinine, urinary protein : creatinine ratio, etc.) and a few candidate urinary biomarkers [MCP-1, NGAL, lipocalin-type prostaglandin D-synthetase (L-PGDS), α1-acid-blycoprotein (AAG/AGP), transferrin (TF), and ceruloplasmin (CP)] in urine samples from 76 SLE patients collected within 2 months of kidney biopsy [reviewed in 7]. These urinary biomarkers were compared with histopathologic features of the kidney biopsy such mesangial expansion, capillary proliferation, crescent formation, wire loops, or fibrosis. Overall, their results indicated that levels of specific urinary biomarkers were increased in active LN and appeared to correlate with distinctive histologic features in renal biopsies. Furthermore, based on the presence of defined urine proteins, the authors could predict specific LN signatures. LN activity signature was defined by a combination of urinary MCP-1, AAG, and CP levels and protein : creatinine ratio while LN chronicity was characterized by NGAL, MCP-1 and creatinine clearance. The combined tests of MCP-1, AAG, TF, creatinine clearance and serum C4 was indicative of a potential biomarker panel for membranous nephritis. However, like NGAL, increased expression of these urine biomarkers is not exclusive to LN. Future studies are likely to highlight the relevance of specific urine biomarker proteins in predicting renal involvement in SLE.
Conclusion
There is considerable evidence that no single biomarker will be sufficient to diagnose, monitor and stratify all patients with SLE. Ideally, new biomarkers should provide information not available from traditional tests. Recent efforts geared towards the discovery and validation of biomarker ‘panels’ or ‘signatures’ of SLE represent an informed approach. Validation studies with endpoints that ensure a true measure of the intended clinical process in diverse cohorts coupled with robust analytical assays and high statistical power to confirm these panels are needed.
References
1. Illei GG, Tackey E, Lapteva L, Lipsky PE. Biomarkers in systemic lupus erythematosus. I. General overview of biomarkers and their applicability. Arthritis Rheum. 2004; 50: 1709–1720.
2. Liu CC, Kao AH, Manzi S, Ahearn JM. Biomarkers in systemic lupus erythematosus: challenges and prospects for the future. Ther Adv Musculoskelet Dis. 2013; 5: 210–233.
3. To CH, Petri M. Is antibody clustering predictive of clinical subsets and damage in systemic lupus erythematosus? Arthritis Rheum. 2005; 52: 4003–4010.
4. Leffler J, Bengtsson AA, Blom AM. The complement system in systemic lupus erythematosus: an update. Ann Rheum Dis. 2014; 73: 1601–1606.
5. Jeltsch-David H, Muller S. Neuropsychiatric systemic lupus erythematosus: pathogenesis and biomarkers. Nat Rev Neurol. 2014; 10(10): 579–596.
6. Cozzani E, Drosera M, Gasparini G, Parodi A. Serology of lupus erythematosus: correlation between immunopathological features and clinical aspects. Autoimmune Dis. 2014; 2014: 321359.
7. Bennett M, Brunner HI. Biomarkers and updates on pediatrics lupus nephritis. Rheum Dis Clin North Am. 2013; 39: 833–853.
8. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997; 40: 1725.
9. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012; 64: 2677–2686.
10. Yu C, Gershwin ME, Chang C. Diagnostic criteria for systemic lupus erythematosus: a critical review. J Autoimmun. 2014; 48–49: 10–13.
11. Isenberg DA, Manson JJ, Ehrenstein MR, Rahman A. Fifty years of anti-ds DNA antibodies: are we approaching journey’s end? Rheumatology (Oxford) 2007; 46: 1052–1056.
12. Kalunian KC, Chatham WW, Massarotti EM, Reyes-Thomas J, Harris C, Furie RA, Chitkara P, Putterman C, Gross RL, Somers EC, Kirou KA, Ramsey-Goldman R, Hsieh C, Buyon JP, Dervieux T, Weinstein A. Measurement of cell-bound complement activation products enhances diagnostic performance in systemic lupus erythematosus. Arthritis Rheum. 2012; 64: 4040–4047.
13. Kao AH, Navratil JS, Ruffing MJ, Liu CC, Hawkins D, McKinnon KM, Danchenko N, Ahearn JM, Manzi S. Erythrocyte C3d and C4d for monitoring disease activity in systemic lupus erythematosus. Arthritis Rheum. 2010; 62: 837–844.
14. Romero-Diaz J, Isenberg D, Ramsey-Goldman R. Measures of adult systemic lupus erythematosus: updated version of British Isles Lupus Assessment Group (BILAG 2004), European Consensus Lupus Activity Measurements (ECLAM), Systemic Lupus Activity Measure, Revised (SLAM-R), Systemic Lupus Activity Questionnaire for Population Studies (SLAQ), Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), and Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI). Arthritis Care Res. (Hoboken). 2011; 63(Suppl 11): S37–46.
15. Hahn BH, McMahon MA. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. (Hoboken). 2012; 64: 797–808.
The author
Anne E. Tebo1,2 PhD
1Department of Pathology, University of Utah School of Medicine, Salt Lake City, UT 84132, USA
2ARUP Institute for Clinical and Experimental Pathology, Salt Lake City, UT 84108, USA
E-mail: anne.tebo@hsc.utah.edu;
anne.tebo@aruplab.com