Biotech Fluidics introduces new flowmeter kit for HPLC pump validation
Biotech Fluidics has developed a new HPLC Pump Validation Kit designed to address longstanding challenges in monitoring highperformance liquid chromatography (HPLC) pump performance. The novel system utilises non-invasive thermal sensor technology to provide real-time validation data, offering laboratory scientists enhanced capabilities for ensuring stable solvent flow critical for accurate chromatographic separations.
Addressing limitations in current technologies
HPLC systems, widely used in pharma-ceutical, clinical and research laboratories, rely fundamentally on consistent solvent flow rates to achieve reliable separation and analysis. Until now, scientists and laboratory technicians have primarily depended on two methodologies for flow rate validation: volumetric flowmeters and Coriolis flowmeters. Volumetric flowmeters, while established in the field, provide integrated measurements over extended time periods and substantial volumes. This approach offers limited insight into moment-to-moment variations and pulsation patterns that can significantly impact chromatographic performance. These limitations can obscure subtle performance issues that might affect analytical precision.
Coriolis flowmeters represent an alternative approach but bring their own set of constraints. Their time resolution capabilities are restricted, potentially missing rapid fluctuations in flow. Additionally, the relatively high cost of Coriolis systems has limited their widespread adoption in laboratory settings where budgetary considerations are often paramount.
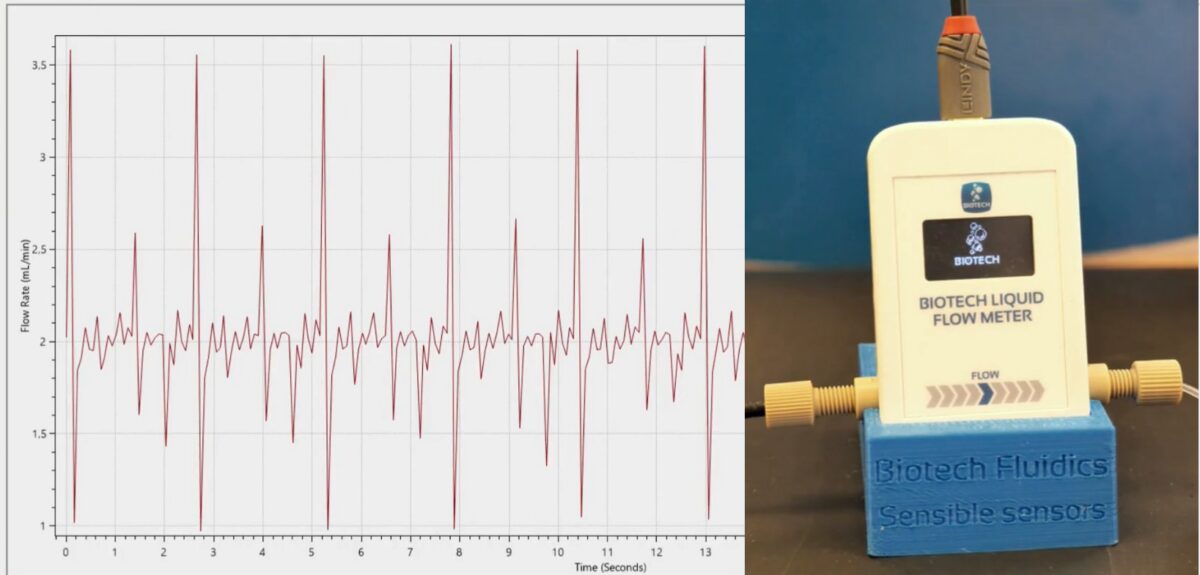
Real-time monitoring capabilities
The newly introduced system from Biotech Fluidics operates on fundamentally different principles, employing non-invasive thermal sensing technology to measure flow rates. This approach enables scientists to observe performance parameters in real time, offering enhanced visibility into pump dynamics.
A significant advantage of the system is its capacity to detect and quantify transient changes in flow properties, including pulsation patterns that might otherwise remain undetected. These capabilities allow for more thorough performance assessment and potentially earlier identification of developing issues before they compromise analytical results.
Automated validation processes
Laboratory efficiency represents a key focus of the new kit. The system accepts raw data generated by the associated PC application and automatically performs all necessary flow calculations, eliminating manual data processing requirements and reducing opportunities for calculation errors.
Further enhancing its utility in regulated environments, the software automatically validates all data and parameters for fitness of use according to predetermined criteria. The system generates comprehensive summary reports that can be stored electronically or printed as hard copy documentation to satisfy quality assurance and regulatory requirements.
Standardised protocols
Recognising the importance of procedural consistency in laboratory settings, particularly in regulated environments, the HPLC Pump Validation Kit includes a comprehensive Standard Operating Procedure (SOP). This documentation details the complete validation protocol, facilitating consistent implementation across different operators and laboratory sites. The standardised approach supports quality assurance initiatives while potentially reducing training requirements for new personnel. Laboratories operating under GLP (Good Laboratory Practice) or GMP (Good Manufacturing Practice) guidelines may find this standardisation particularly valuable for maintaining documentation compliance.
Applications beyond validation
While primarily designed for pump validation purposes, the system offers additional functionality for ongoing performance monitoring. Laboratories can utilise the technology to observe real-time transient changes in flow characteristics, providing insights that might inform preventative maintenance schedules or troubleshooting procedures.
This expanded capability potentially extends the utility of the system beyond periodic validation exercises to support continuous monitoring paradigms, allowing scientists to maintain optimal chromatographic performance through enhanced visibility of system dynamics.
Biotech Fluidics positions itself as a leading supplier of fluidic system solutions, liquid transfer components, degassing systems, and innovative laboratory technology for instrument developers, manufacturers, and distributors globally.
For more information, visit: https://shorturl.at/ZbW7Q
Digital issue: Please click here for more information