Bruker expands infectious disease diagnostics portfolio at ESCMID 2025
Bruker Corporation has announced significant expansions to its infectious disease testing solutions at the ESCMID Global conference in Vienna, including a broader assay menu for its BeGenius® molecular diagnostics system and improvements to its microbial identification platforms.

Bruker’s BeGenius sample-to-answer molecular diagnostics system
Enhanced molecular diagnostics capabilities
The company revealed an expanded portfolio of validated PCR assays on its BeGenius sample-to-answer molecular diagnostics system. This automated PCR platform now offers an extensive range of in-vitro diagnostics tests spanning multiple clinical areas, including transplant pathogen monitoring, gastrointestinal infections, sexually transmitted diseases, meningitis, encephalitis, healthcare-associated infections and blood-borne viruses.
According to Bruker, the BeGenius system can process up to 72 tests during an 8-hour shift and is capable of handling diverse human sample types whilst running multiple different assays simultaneously. The system’s compact footprint and open channel capabilities for customer assays make it a versatile, medium-throughput solution for clinical laboratories.
New software for hospital infection control
The new IR Tracker™ software for the IR Biotyper® system aims to improve hospital outbreak and hygiene control protocols. This frontline solution accelerates the detection of suspected outbreak isolates by providing clear, colour-coded reports on microbial strain relatedness and clonality.
Bruker describes this as an “effective early warning system” that facilitates “fast and cost-effective detection or rule-out of potential outbreaks directly in the hospital.” The company suggests this approach minimises time-to-result and reduces the number of healthcare-associated infection samples requiring more time-consuming next-generation sequencing reflex-testing.
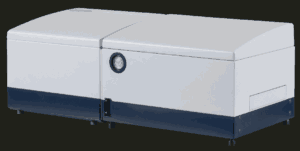
Bruker’s IR Biotyper system
Automated sample preparation improvements
The updated MBT Pathfinder® adds time-saving functionality to sample preparation on the MALDI Biotyper platform, which Bruker refers to as the “gold standard” in microbial species identification. The system’s new reagent deposition feature eliminates manual intervention through fast, precise, contactless deposition of formic acid and HCCA matrix on prepared culture samples.
This automation is designed to remove repetitive tasks and free up laboratory personnel for more value-added activities, bringing time and cost savings to laboratories in a flexible, modular system.
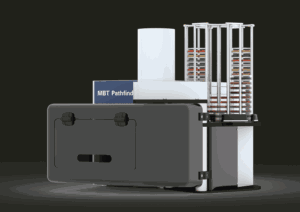
Bruker’s MBT Pathfinder
Commitment to innovation in microbiology
Dr Wolfgang Pusch, President of the Bruker Microbiology & Infection Diagnostics division, commented on the announcements: “At Bruker, we are committed to continuous innovation in our routine microbiology and infectious disease testing portfolio, developing world-leading, future-proof solutions that accelerate patient diagnostics, simplify operations and improve sustainability in the lab. At ESCMID Global we showcase the breadth of our portfolio, the ease of product use, and the fast time-to-result that we offer in microbial identification, hygiene testing and molecular diagnostics.”
The company says these enhancements are part of its broader mission to enable scientists to make breakthrough discoveries and develop new applications that improve human health through high-performance scientific instruments and analytical solutions.
- For more information, visit: bruker.com




