Cherwell introduces TwistLock plates for enhanced microbial monitoring security
UK-based cleanroom microbiology specialist Cherwell has launched a new range of prepared media plates featuring secure locking technology designed to strengthen contamination control and data integrity in pharmaceutical environmental monitoring programmes.
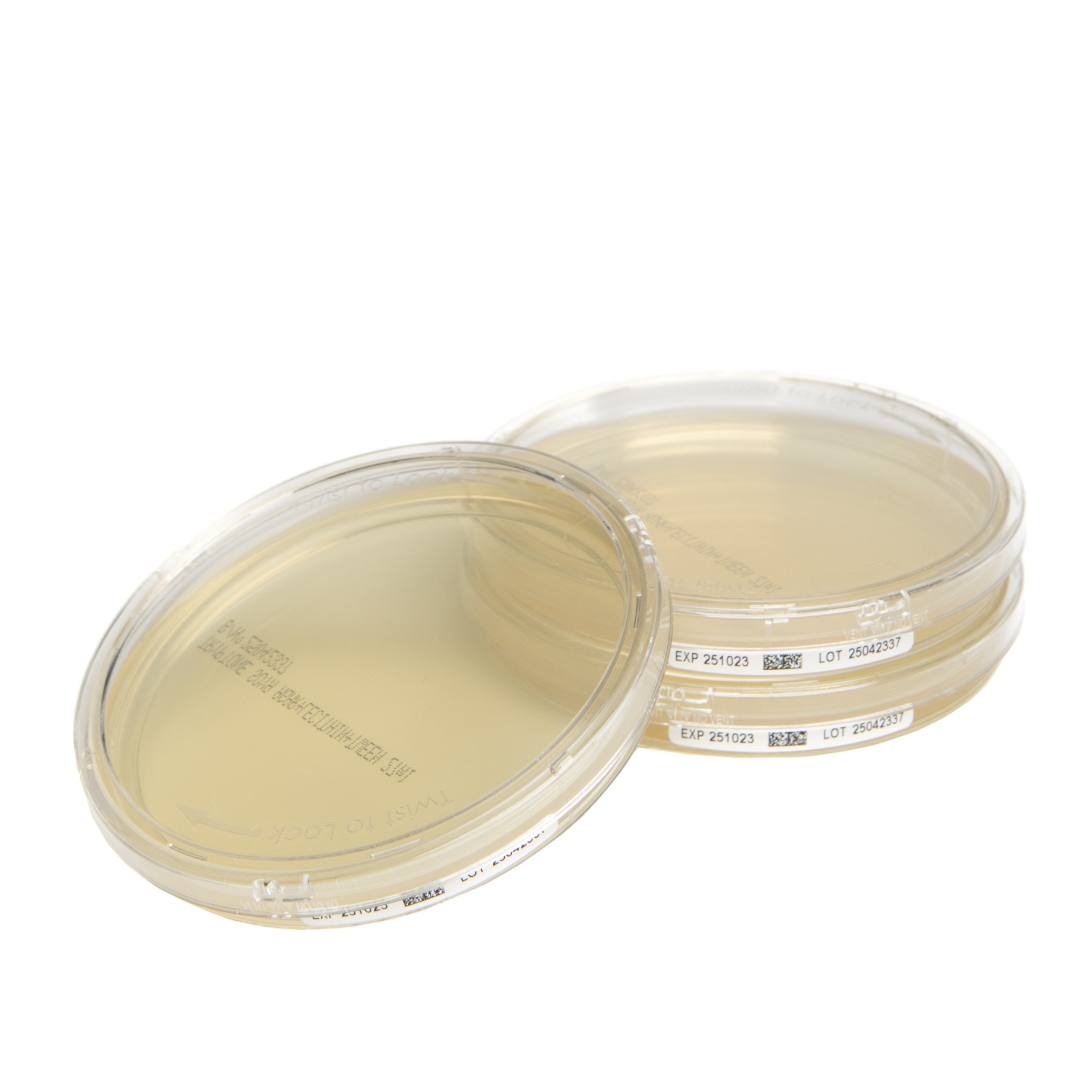
The Redipor® TwistLock plates incorporate a secure locking lid mechanism combined with GS1 DataMatrix barcoding on side-labels, addressing critical requirements for sample security during transport and handling within sterile manufacturing environments. This advancement responds to increasingly stringent regulatory demands outlined in GMP Annex 1 and ICH Q9 principles.
Advanced contamination protection strategy
The TwistLock technology creates a secure seal that prevents contamination whilst protecting sample integrity during transport within and between facilities. This mechanical innovation addresses a fundamental challenge in microbial environmental monitoring where sample integrity can be compromised during routine handling procedures.
“Meeting Annex 1 and other key regulatory requirements by ensuring data integrity and reducing contamination risk in sterile product manufacturing processes is one such key need,” explained Yoggya De Silva, Microbiology Product Specialist at Cherwell. “The launch of our new locking lid Redipor TwistLock prepared media plates provides an answer to the demand for ever-increasing vigilance, and building resilient and reliable microbial EM programmes.”
Digital integration capabilities
The plates feature GS1 DataMatrix barcodes that enable seamless integration with laboratory information management systems (LIMS) and electronic batch records. This digital connectivity supports automated colony counting procedures and enhances traceability throughout the analytical workflow, addressing regulatory requirements for comprehensive documentation and audit trails.
Manufacturing specifications and availability
Like Cherwell’s standard Redipor prepared media range, the TwistLock plates are manufactured triple-wrapped and gamma irradiated in both Petri and contact plate formats. This processing ensures sterility whilst extending shelf-life to six months under appropriate storage conditions.
The plates are available with customisation options including varying media formulations, fill volumes, and packaging formats to accommodate specific laboratory requirements.
Manufacturing will occur across multiple sites including the UK, United States, and Netherlands through Cherwell’s parent company AnalytiChem, ensuring global supply chain security.
Regulatory compliance framework
The TwistLock plates align with GMP Annex 1 guidelines and ICH Q9 quality risk management principles, supporting pharmaceutical manufacturers in developing robust contamination control strategies. The enhanced security features are particularly relevant for facilities implementing risk-based environmental monitoring programmes where sample integrity documentation is critical for regulatory compliance.
The user-friendly design aims to streamline laboratory workflows by reducing handling errors and minimising costly retesting procedures, supporting operational efficiency in high-throughput analytical environments.
For more information, visit: https://shorturl.at/1V41f
Digital issue: Please click here for more information