Complete HPV detection and typing in cervical cancer prevention
Cervical cancer is a major burden worldwide with significant mortality, especially in developing countries. Human papillomavirus (HPV) analysis is gaining ground as the primary screening modality for the early diagnosis and prevention of cervical carcinoma. Direct pathogen detection allows an infection to be identified before cell changes have even taken place. Thus, interventional measures can be applied before the cancer even develops, helping to reduce the overall incidence and mortality rates. The EUROArray HPV molecular diagnostic microarray provides highly sensitive detection and typing of all known high- and low-risk anogenital HPV in one reaction. With fully automated data analysis it is particularly well suited to the high-throughput requirements of routine screening.
Human papillomaviruses
Human papillomaviruses are uncoated double-stranded DNA viruses which infect epithelial cells of the skin and mucous membranes. They are transmitted by sexual contact. Infection is assumed to occur via tiny lesions in the basal cells of the epithelium. Thus, the most frequent place of infection is the transformation zone of the cervix, where dividing basal cells lie near to the surface. The size of the cells, their histology and the duration of the lesion can influence the number of cells infected. The course and outcome of the infection depends on the HPV type, the anatomy of the infection site and the differentiation status of the host cells.
Infections with HPV are always local and are not accompanied by viremia. Following infection, the viral DNA is replicated in the host cell nuclei. Viral proteins produced in the infected cells can trigger uncontrolled tumour-like growth of the cells. This is, depending on the infecting HPV subtype, mostly benign, leading to warts at the site of infection. However, some HPV types can induce malignant changes, particularly cervical cancer. A significant proportion of vaginal, penile, anal and head and neck carcinomas are also assumed to be caused by HPV infection.
HPV are the most frequent sexually transmitted viruses. The worldwide prevalence of HPV infection is estimated to be 2 to 44% in women and 4 to 45% in men, with regional variations depending on culture and the corresponding sexual activity. Viral transmission from mother to newborn during birth can also occur, even with subclinical infections. HPV infection does not lead to life-long immunity and reinfection with the same virus is possible.
HPV subtypes
Around 130 types of HPV have so far been described of which 30 infect exclusively the skin and mucous membranes in the anogenital area. HPV are divided into two groups according to their oncogenic potential. High-risk HPV cause cervical carcinoma. Low-risk HPV alone do not induce tumours, but cause non-malignant tissue changes. Concurrent infections with multiple HPV subtypes are common and known to increase the risk of malignant cell transformations.
Of the high-risk anogenital types, HPV 16 and HPV 18 are responsible for around 70% of cervical carcinomas. HPV 16 is found in 50 to 60% of cases and HPV 18 in 10 to 20%. Other types classified as high-risk by the WHO are 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 66. Types 26, 53, 68, 73 and 82 have also been detected in cervical carcinoma and should be considered as high-risk types.
Of the low-risk types, HPV 6 and 11 are the main causative agents of genital warts (condylomata acuminata, fig warts). Further low-risk types are 40, 42, 43, 44, 54, 61, 70, 72, 81 and 89.
Cervical carcinoma
HPV infection is a prerequisite for the development of cervical carcinoma. However, HPV infection does not necessarily lead to cancer. Most infected women eliminate the virus within two years. If the virus remains detectable for longer than 18 months, the infection is considered to be persistent. A persistent infection, in particular with a high-risk HPV subtype, increases the risk of developing cervical carcinoma by around 300-fold.
HPV infections are often asymptomatic and tend to remain unnoticed. The initial stages of cervical carcinoma also proceed without pain, and the only symptom may be light bleeding. With increased tumour size, the cancer manifests with a blood-tinged, sweet smelling discharge.
Around 528,000 new cases of cervical carcinoma occur annually worldwide, making it the fourth most frequent cancer in women after breast, colorectal and lung cancers. It is also the fourth most common cause of cancer mortality, causing approximately 266,000 deaths in 2012 (International Agency for Research on Cancer).
In the early stages, treatment involves removal of the altered tissue by conisation. In later stages of the disease, the uterus and surrounding tissue must be removed.
Role of HPV detection and typing
Along with the current diagnostic gold standard, the Papanicolaou (Pap) test, HPV direct detection plays an important role in the early diagnosis of cervical carcinoma. In contrast to the Pap test, which is used to investigate cervical cells for pathological changes, PCR-based methods detect viral nucleic acids directly, and can thus identify an HPV infection at a very early stage before morphological cell changes have even occurred. Moreover, while the Pap test is based on subjective evaluation, HPV detection represents an objective as well as extremely sensitive test method.
In HPV screening it is crucial to differentiate between high- and low-risk types and also to discriminate between different high-risk viruses. A positive result for high-risk HPV indicates an increased risk for cervical carcinoma, which can then be minimized by more frequent follow-up examinations to detect morphological cell changes at an early stage. A positive result for low-risk HPV can help to clarify uncomfortable and embarrassing symptoms for patients. Since low-risk HPV can also cause mild dysplasia, HPV subtyping is also useful for excluding a high-risk HPV infection and a corresponding risk of cervical cancer in these cases. Women who are HPV negative can forgo Pap smears for a longer time interval, based on the recommendations of the respective professional societies.
The PCR detection strategy is a critical aspect of direct HPV analysis. Tests with primer or probe systems based on conserved genes like L1 may yield false negative results in some cases due to loss of these genes during integration of the viral DNA into the host DNA. The highest possible detection sensitivity is achieved using the viral oncogenes E6/E7. Detection of variable sequences in these genes enables differentiation of the different HPV subtypes.
Microarray for complete HPV typing
A standardized microarray based on PCR detection of E6/E7 has been developed for complete HPV typing in routine diagnosis. Using an extensive panel of specific primers and probes, the EUROArray HPV detects all thirty genitally relevant HPV subtypes in one test, distinguishing eighteen high-risk subtypes that may trigger cancer (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82) and twelve low-risk subtypes that cause benign warts (6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81, 89). Multiple infections are reliably identified, and primary and persistent infections can be differentiated.
Simple procedure with automated evaluation
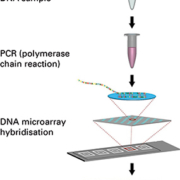
The EUROArray procedure (Figure 1) is extremely easy to perform and does not require any in-depth molecular biology knowledge. DNA prepared from patient cervical smear samples is first amplified by a single multiplex polymerase chain reaction (PCR). The fluorescently-labelled PCR products are then incubated with biochip microarray slides (Figure 2) containing immobilized complementary DNA probes. Specific binding (hybridization) of the PCR products to their corresponding microarray spots is detected using a specialized microarray scanner.
In contrast to manually evaluated tests, the results are evaluated (Figure 3) and interpreted fully automatically by user-friendly software (EUROArrayScan). A detailed result report (Figure 4) is produced for each patient and all data is documented and archived. Meticulously designed primers and probes, ready-to-use PCR components and integrated controls all contribute to the reliability of the analysis. The entire EUROArray process from sample arrival to report release is IVD validated and CE registered, supporting quality management in diagnostic laboratories.
Conclusion
As evidence mounts about the efficacy of HPV testing for primary cervical cancer screening, multiplex microarrays are poised to become a major tool in prevention programmes worldwide. The EUROArray HPV, in particular, is ideally positioned for high-throughput HPV screening, providing fast and sensitive detection of all high- and low-risk anogenital HPV types combined with fully automated data analysis.
The author
Jacqueline Gosink, PhD
EUROIMMUN AG
Seekamp 31
23560 Luebeck
Germany
E-mail: j.gosink@euroimmun.de