Diagnosis and management of subarachnoid hemorrhage
If a patient has a suspected subarachnoid brain hemorrhage (SAH), time is of the essence. CLI chatted to Dr Suneesh Thilak (New Cross Hospital, Wolverhampton, UK) to find out about the role that imaging plays in diagnosis, securement and subsequent patient management of SAH.
What is subarachnoid hemorrhage?
Subarachnoid hemorrhage (SAH) is a neurovascular emergency due to a bleed into the subarachnoid space, which is a space between the brain and the tissues covering the brain. It is most commonly due to a rupture of an aneurism causing a significant neurovascular emergency. Almost 80 to 85% of cases are due to spontaneous aneurism rupture. The incidence of SAH is around 8 in 10 000 people per year, it is slightly more common in females than males, and mostly affects patients of the age group of 50 to 60 years. Unfortunately, this is generally when people are at the height of their productivity, and so ill health at this time causes a significant economic burden to the family, to society and overall to the economy.
Why is it important to diagnose SAH?
Prompt diagnosis of subarachnoid hemorrhage (SAH) is crucial due to its significant morbidity and mortality. The adage “time is brain” underscores the importance of immediate recognition and intervention. Early diagnosis facilitates timely treatment of the hemorrhage, which is essential to prevent further neurological damage and reduce morbidity. A significant proportion of SAH-related deaths occur within the first week after the initial bleed, highlighting the need for urgent diagnosis and management.
How is SAH diagnosed and what is the role of imaging?
While SAH typically presents acutely, there are instances where unruptured aneurysms may be detected incidentally during investigations for other conditions. Most patients come in with an acute presentation of a sudden and severe headache (also called a thunderclap headache), which the patient might not have experienced before. A non-contrast computed tomography (CT) scan is the primary investigation for suspected SAH. Non-contrast CT scanning has a very high sensitivity for detecting SAH, approaching 100% within the first 6 hours of symptom onset, with sensitivity decreasing slightly thereafter. Although the sensitivity of the non-contrast CT scan remains high, there is a risk of missing SAH in some cases. If SAH is suspected despite a negative initial CT scan, a lumbar puncture (LP) is performed to analyse cerebrospinal fluid (CSF). Xanthochromia, a yellowish discoloration of the CSF, is indicative of SAH and typically becomes detectable after 12 hours. However, LP carries a small risk of complications (e.g. rebleeding, herniation) in patients with SAH, and careful consideration is needed. Delays in diagnosis can occur due to various factors, including atypical symptoms, misdiagnosis as other conditions (e.g. migraine), and logistical issues such as patient transport or long wait times. Although the classic presentation is thunderclap headache, symptoms including a low GCS score, seizures and also a focal neurological deficit are all associated with SAH.
What is the role of imaging in the management of SAH?
Imaging is the first stage in the diagnosis of SAH, but then we also want to understand the etiology of the hemorrhage. We want to know if it is due to an aneurysm or some other vascular malformation, and for this, we use a CT angiogram to characterize the reason for the SAH. From this, we can do a three-dimensional reconstruction of the arteries and pinpoint where the bleed is occurring, its size, etc.
After diagnosis, the next important management step with SAH is monitoring over the next 24 hours, as studies show that within 24 hours, there’s a high risk for rebleeding. If the patient’s level of consciousness deteriorates, it could signal another neurological event. Possible causes include rebleeding, hydrocephalus (a buildup of fluid in the brain), vaso-
spasm (narrowing of the blood vessels), cerebral edema (swelling in the brain), seizures, electrolyte imbalances, or infections. Given the dynamic nature of SAH and the potential for rapid neurological changes, repeat CT scans are often necessary. These serial imaging studies provide crucial information about the progression of the hemorrhage and the development of potential complications. The gold standard diagnostic is digital subtraction angiography (DSA), but this technique also helps with treatment, as we can perform coiling of the aneurysm at the same time. This technique involves a catheter being introduced into the femoral artery and guided by imaging to the site of the aneurysm. Then, coils of platinum wire are passed through the catheter to fill the aneurysm, which stops blood flowing into the aneurysm and stops the bleeding. Coiling is minimally invasive, and although there is a slight risk of recurrence, studies have shown that overall morbidity and mortality are better compared to surgical securement of the aneurysm. Surgical securement involved a craniotomy where the skull was opened, the surgeon had to find the aneurysm/hemorrhage and clamp it.
While DSA and CT angiography are essential for diagnosis and treatment planning, other imaging modalities also play a role in SAH management. MRI can be used for patients who are allergic to the contrast medium and can also be valuable in cases where CT is inconclusive.
Additionally, perfusion CT is also done, Perfusion CT is used to evaluate cerebral blood flow and identify areas of reduced perfusion (ischemia), which can occur due to vasospasm or other complications of SAH. Transcranial Doppler ultrasound can also be performed at the bedside, which is another modality, although still with less sensitivity than CT. Once the aneurysm is secured, patients are managed in a neurocritical care center (specialized units with the expertise and resources to manage critically ill neurological patients, including those with SAH. The key features are 24/7 availability of neurosurgeons, neurointensivists, and advanced imaging capabilities). Studies have shown that management in a neurocritical care center leads to better outcomes for patients with SAH. This means that anytime a patient arrives, the CT scan can be done along with early securement of the aneurysm. Following aneurysm securement, patients are admitted to the ICU or a postoperative ward based on their Glasgow Coma Scale (GCS) score. There, the patients are monitored carefully over the next three days for any sign of rebleed or early brain injury. If there is any decrease in the level of consciousness of the patient or development of new symptoms, the patient will immediately have another CT scan, and the complication can be managed. For example, in the event of vasospasm (which is a narrowing of the blood vessel causing decreased perfusion), we would do DSA and balloon angioplasty to open up the blood vessel. In conclusion, CT and CT angiography are indispensable tools in the diagnosis, management, and ongoing monitoring of subarachnoid hemorrhage.
Are there any advances on the horizon or that would improve SAH diagnosis and management?
If we were to use CT perfusion scans regularly, we could diagnose delayed cerebral ischemia at an earlier stage. Also, in high-volume care centres, CT perfusion often complements multi-modal monitoring, invariably with bedside monitoring, including transcranial Doppler ultrasound and biomarker monitoring. This includes, for example, S100B or neuron-specific enolase (NSE), checking lactate/pyruvate ratios, which gives us an indication of whether anaerobic respiration is occurring, which is a sign of cerebral hypoxia or ischemia. Hence, multi-modal monitoring builds a more comprehensive picture of what is happening in the brain and improves outcomes compared to examining the results from a single investigation.
Also on the horizon is the use of machine learning and artificial intelligence, which will help to analyse radiological images and identify issues that a human might miss. Other potential applications of machine learning, such as predicting vasospasm or identifying patients at higher risk of complications.
Advances in MRI, such as time-of-flight magnetic resonance angiography, its non-invasive nature and ability to visualize blood flow without contrast, will be useful for improving outcomes in patients who are allergic to or have anaphylactic reactions to the contrast agent needed for CT angiography.
One crucial aspect of achieving the best outcome in the event of SAH is to minimize transfer time to a neurocritical care centre. As soon as SAH is suspected – or if paramedics encounter a patient with the classic headache symptoms – the patient needs to be transferred immediately to a neurocritical care centre where there is availability of specialized team members consisting of neurointensivists, Interventional neuroradiologists and Neurosurgeons available round the clock, where an urgent CT scan can be performed, and the aneurysm secured as quickly as possible, along with careful monitoring during the next 24-hour period or longer. There are five or six such centres in the UK, and at times transfer can be by helicopter to minimize delay.
In very remote areas, telemedicine can be useful for consulting neurology experts to avoid delays in deciding whether a patient needs to be transferred to a neurocritical care centre. Moreover, advances in robotics will ultimately allow telesurgery or remote surgery, which is where robotic technology and wireless networking will enable a surgeon to operate on a geographically distant patient, which again will overcome the problems associated with lengthy transfer times and improve outcomes.
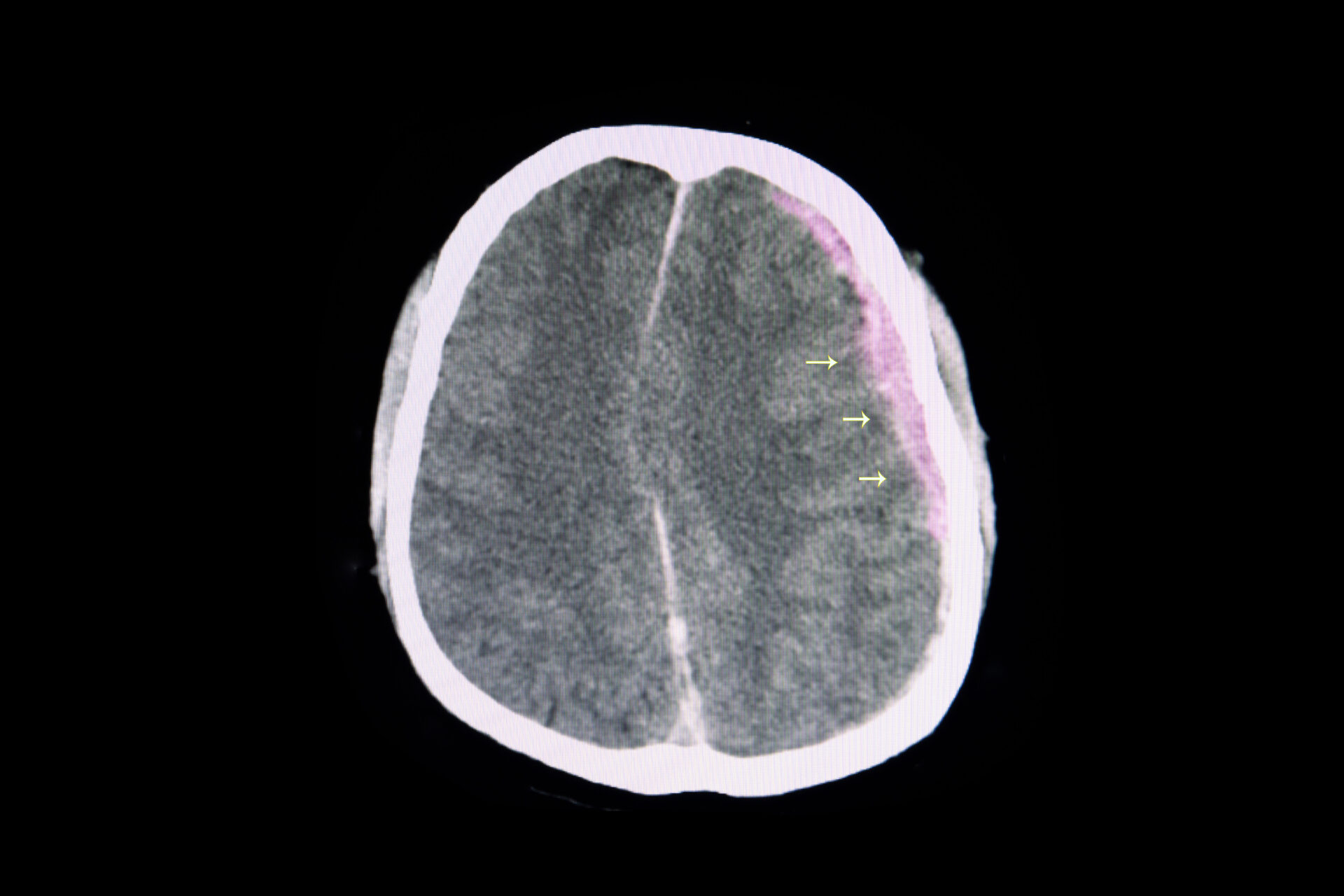
CT scan of brain showing subarachnoid hemorrhage
(Adobestock.com)
The interviewee
Dr Suneesh Thilak DNB Anaes, IDCCM, MPhil, EDAIC, EDIC, AFICM Consultant ITU and Anaesthesia
New Cross Hospital, Royal Wolverhampton NHS Trust, Wolverhampton, UK
Email: suneesh.thilak@nhs.net
For further information see: Thilak S, Brown P, Whitehouse T et al.
Diagnosis and management of subarachnoid haemorrhage.
Nat Commun 2024;15(1):1850
( https://www.nature.com/articles/s41467-024-46015-2 ).