First Swiss public health institute deploys cloud-based digital pathology
University of Bern makes landmark move to AWS-powered HALO AP platform for diagnostic tissue analysis
Pioneering cloud deployment transforms diagnostic workflow
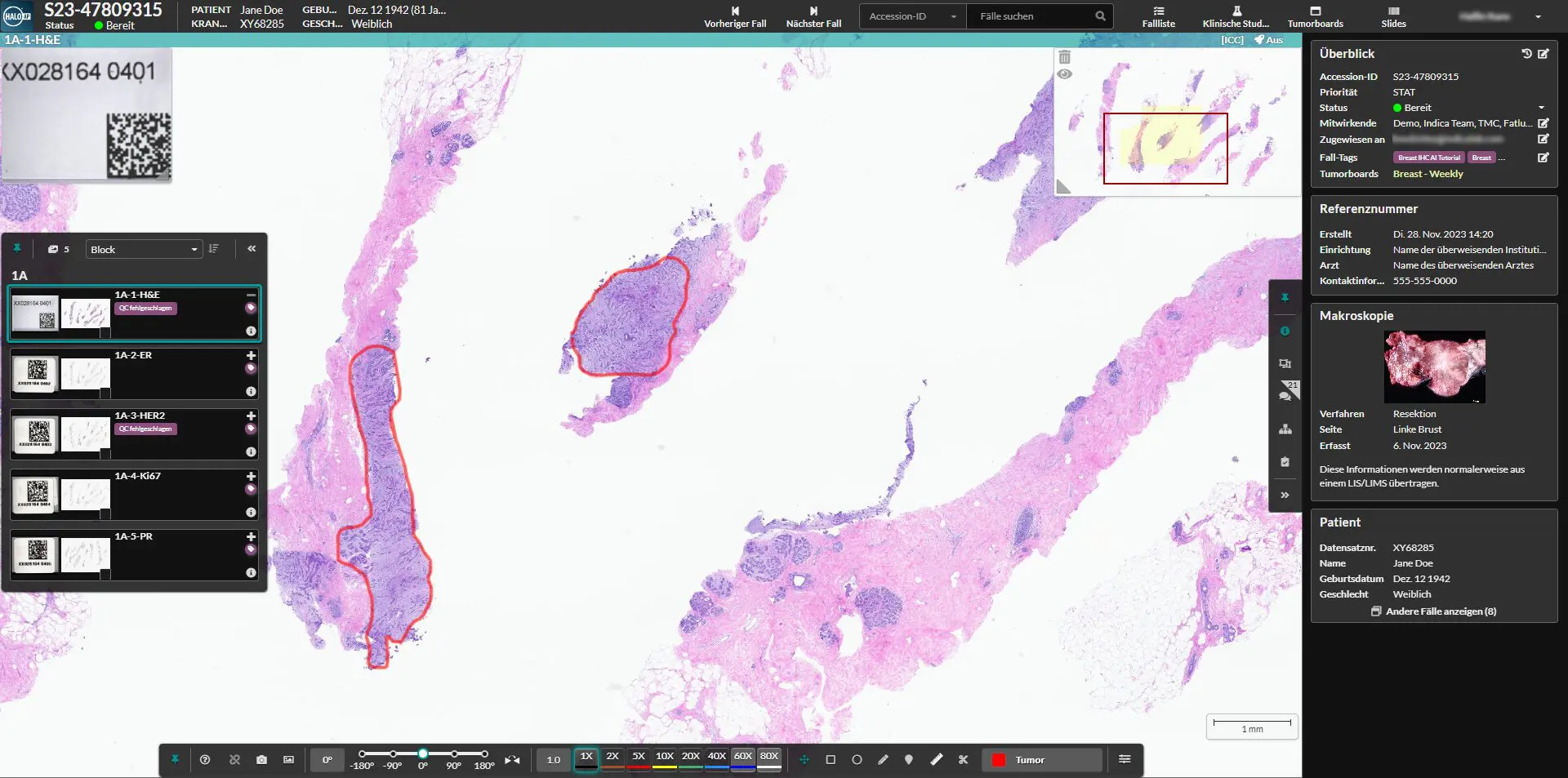
The Institute of Tissue Medicine and Pathology at the University of Bern has become Switzerland’s first public health institution to receive approval for using cloud-based digital pathology for diagnostics. This significant development sees the deployment of Indica Labs’ HALO AP platform on Amazon Web Services (AWS), marking a transformative approach to tissue analysis and pathology diagnostics in the Swiss healthcare ecosystem.
The milestone implementation, announced on 11 December 2024, represents the first time a Swiss public health institution has received local authority approval to utilize AWS for managing personal healthcare data in diagnostics. This approval from Bern Canton’s data privacy office enables the Institute to store encrypted patient data in AWS Zurich region, providing a scalable and secure foundation for advanced pathology services.
Technical infrastructure enhances diagnostic capabilities
HALO AP, developed by Indica Labs, delivers comprehensive digital pathology capabilities designed to address the multifaceted requirements of modern pathology laboratories. The platform integrates a suite of specialized tools for image analysis, diagnostics, and collaboration, with emphasis on interoperability across various scanners and file formats.
A distinguishing feature of this implementation is its cloud-based architecture, which promises enhanced scalability and cost-effectiveness compared to traditional on-premise solutions. The Institute worked extensively with AWS partner foresite AG to navigate the complex regulatory requirements, developing comprehensive documentation for data protection compliance.
“The implementation of HALO AP for digital tissue medicine at the University of Bern represents a major step towards streamlining diagnostic workflows and preparing for a future with AI-enabled pathology,” said Prof. Dr. Aurel Perren of the Institute of Tissue Medicine and Pathology at the University of Bern. “We anticipate that deploying digital pathology in a secure AWS environment tailored to our institute’s needs will positively impact turnaround time, diagnostic quality and will ultimately contribute to improving patient care.”
Technical compliance underpins medical data security
The deployment incorporates rigorous technical safeguards to ensure compliance with European medical device regulations. HALO AP is CE-IVDR marked for in-vitro diagnostic use in Europe, the UK, and Switzerland, though it remains for research use only in the USA without FDA clearance for clinical diagnostics. The platform’s architecture includes built-in compliance with FDA 21 CFR Part 11, HIPAA, and GDPR requirements, establishing a robust framework for secure medical data management.
The technical integration process involves Indica Labs’ Cloud Services team working directly with the Institute to optimize the platform’s implementation within existing clinical workflows and IT infrastructure. This collaborative approach aims to maximize the diagnostic benefits while ensuring seamless transition from conventional pathology processes.
Cloud architecture offers flexibility and performance advantage
The AWS-powered deployment provides the Institute with infrastructure advantages that traditional on-premise solutions cannot match. Cloud technology’s inherent scalability allows for dynamic resource allocation that can accommodate varying workloads, while the geographic specificity of data storage in the AWS Zurich region addresses data sovereignty requirements.
“We are thrilled to work with the Institute of Tissue Medicine and Pathology at the University of Bern,” said Eric Runde, COO of Indica Labs. “This partnership will create a truly bench-to-bedside approach to pathology that will improve patient care and diagnostic efficiency. By utilizing our Cloud Services team for this deployment, the Institute will have a truly scalable digital pathology solution with the support they need to run an efficient and patient-centered service.”
Impact on pathology practice and research
The implementation represents a significant advancement for pathology diagnostics in Switzerland, potentially establishing a template for other institutions considering similar digital transformations. By integrating digital pathology into its diagnostic workflow, the Institute positions itself to leverage future developments in artificial intelligence and computational pathology.
For practising pathologists, the platform offers enhanced collaboration capabilities, enabling consultation across geographical boundaries and facilitating second opinions. The integration of image analysis tools within the diagnostic workflow may increase diagnostic precision while potentially reducing interpretation time for complex cases.
The platform’s flexibility extends to research applications, potentially bridging the gap between clinical practice and translational research within the same institutional framework. This unified approach to pathology data management could accelerate the development and validation of novel diagnostic approaches and biomarkers.
As digital pathology continues to mature as a discipline, this landmark deployment at the University of Bern demonstrates how cloud-based infrastructure can overcome traditional barriers to adoption, providing a technical foundation for next-generation tissue diagnostics that balances security, performance, and clinical utility.