External Quality Assessment (EQA) for trace element measurements in clinical laboratories
External Quality Assessment (EQA) is the cornerstone of quality assurance and method validation in clinical testing labs in the UK, ensuring that the results of patient investigations are reliable and comparable wherever they are produced. In this article we focus specifically on EQA for laboratories performing trace element measurements, although many of the points are applicable to the wider pathology areas.
by S.-J. Bainbridge and Dr C. F. Harrington
Introduction
External Quality Assessment (EQA), also termed proficiency testing (PT), involves the regular distribution of test materials to participating laboratories so that they may evaluate their analytical performance against a peer-group, detect any accuracy or other problems that may develop with the assay and so improve the results that they produce. The key elements that differentiate EQA from PT include: education and support; identification of method poor performance; and method evaluation [1].
Historically clinical science was one of the first disciplines to realize the usefulness of EQA and take steps to implement schemes that would be of use in the hospital laboratory. The first proficiency survey of UK clinical pathology laboratories was reported in 1953 and revealed a wide spectrum of results for the common tests [2]. Further surveys in the 1950s and 60s confirmed the need for regular PT. In 1969, the National Quality Control Scheme was initiated by the Wolfson Research Laboratories, Birmingham and involved the distribution of specimens every 14 days [2]. This is now known as the UK National External Quality Assessment Scheme (UKNEQAS) and is responsible for about 30 different schemes.
In 2013, the importance of EQA in the NHS pathology services was emphasized by Dr Ian Barnes in a Department of Health review into quality assurance [3]. The review assessed current NHS quality assurance frameworks and governance mechanisms for pathology services. It gathered a diverse range of evidence: examining expectations of pathology services; identifying areas for improvement; and recommending a system-wide way forward. It recommended strengthening and standardizing the current quality assurance structures that are in place, which are based on the Royal College of Pathologists (RCPath) Joint Working Group for Quality Assessment (JWGQA), which co-ordinates and oversees the standards and performance of EQA schemes for all schemes regardless of provider.
EQA for trace elements
The Trace Elements External Quality Assessment Scheme (TEQAS), which is part of UKNEQAS but based in Guildford, UK, was established in 1979 with distribution of specimens on a monthly schedule to UK hospital laboratories measuring copper and zinc in serum. During the next five years the scheme developed with inclusion of other participants and the introduction of additional analytes and specimen types. Following an international conference on Aluminium and Renal Disease in 1986, a two year arrangement was established with the EU Commission to fund participation in the serum aluminium programme for European laboratories involved with the monitoring of patients with chronic renal failure. An outcome of this work was the realization that analytical standards of performance for this measurement were very poor. In collaboration with the UK Department of Health it was proposed that the scheme should be linked to UKNEQAS in order to provide a mechanism for referral of poor performers to the Clinical Chemistry Advisory Panel. This link was formally established by the Advisory Committee on Analytical Laboratory Standards in 1988. The aims of TEQAS are consistent with the intentions of UK NEQAS, to:
- Provide professionally-led and scientifically-based schemes with a primarily educational objective.
- Provide regular distributions of specimens.
- Provide rapid feedback of performance.
- Support participants where problems occur.
- Stimulate the overall improvement in performance among all participating laboratories.
The main TEQAS scheme provides EQA for: Al, Cr, Co, Cu, Se and Zn in serum; As, Cd, Cr, Co, Pb, Mg, Mn, Hg, Se, Tl and Zn in whole blood; and As, Cd, Cr, Co, Cu, Fe, Pb, Mn, Hg, Ni, Tl and Zn in urine. This operates on a monthly cycle, with the results from the measurement of two specimens being returned on-line on the last day of each month. The report is then available five days later to download. Two smaller schemes providing Al in dialysis fluid and Cu and Fe in solid matrices are also available, but have a more limited number of participants.
The main steps in the EQA process
For a better appreciation of the overall EQA scheme it is useful to divide it into a number of process-based areas as shown in Figure 1. The activities that comprise these areas are also shown. Some of these areas come under particular focus as part of ISO 17043:
Design
Appropriate design of the PT scheme ensures that participants will have paid for a service that provides high quality comparable test items that are representative of patient samples they would normally expect to analyse. These test items will have undergone thorough assessment procedures in accordance with acceptable statistics to ensure a homogenous and stable test item.
Materials
The participants can be assured that the materials used in the production of the PT samples have been ethically and legally obtained and that the competence of the suppliers have been evaluated and verified to ensure their products or services do not affect the quality of the PT scheme.
Evaluation
ISO 17043:2010 ensures the evaluation of the participants performance is conducted fairly and consistently guaranteeing they receive an accurate evaluation calculated from the use of robust statistical methods.
Knowledge and Experience
In addition to all this ISO 17043:2010 ensures that the operations of the PT scheme are carried out by personnel that have the training, skills and competence necessary to professionally carry out their assigned tasks. Participants can feel secure in knowing that they have access to the specialist knowledge and expertise in the field of trace element testing to be able to discuss any concerns they may have.
Establishment of performance criteria
Measurements of performance are based on deviations of results from target values, which are used to calculate a Z-score. As EQA has developed, various organizations have produced documents that summarize best practice. Those from authoritative international bodies include:
- ISO 17043 (Conformity assessment – General requirements for PT).
- ISO 13528 (Statistical methods for use in PT by interlaboratory comparisons).
- IUPAC (The international harmonized protocol for the PT of analytical chemistry laboratories) [4].
All these documents recommend that assessment of performance should be based upon calculation of a Z-score (or a derivative which takes uncertainty into consideration).
The Z-score is calculated as:
x-X/ SDPT
where x = laboratory result,
X = target value, and
SDPT = standard deviation for PT (also represented as σ)
The ‘standard deviation for PT’ is set by the scheme organizer but should ideally be a value that will allow the score to demonstrate whether or not the performance is fit for the purpose for which the assay is being used. It is recommended that this value be set so that a Z-score of up to ±2 indicates acceptable performance and a score of more than ±3 indicates unsatisfactory performance.
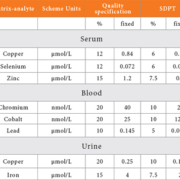
In the TEQAS scheme, we have used quality specifications based on biological variation for the ‘standard deviation for PT’ and the determination of these quality specifications has been published [5]. For assays where there is insufficient data to prepare specifications in this way we have produced values that are related to performance within the scheme during recent years.
The quality specifications and their corresponding SDPT for some illustrative elements are shown in Table 1. These are presented as either a percentage of the target value or a fixed value depending on the concentration of the target value, and the one used is whichever is the greater. This allows for the increase in imprecision at low concentrations and conforms to a ‘funnel’ shape.
Scheme accreditation
Accreditation is fast becoming a preferred mechanism for delivering confidence in UK Healthcare and with the application of BS EN ISO 15189:2012 into Medical Laboratories and its requirement for the laboratories to seek confirmation for confidence in their results, the need for EQA schemes in the relevant fields of medical laboratories is ever increasing. Participation in a suitable scheme can be an effective way of demonstrating the laboratories’ technical competence. ISO 15189:2012 requires laboratories to evaluate their PT providers and a recognized acceptable basis of their evaluation recommended by the UK Accreditation Service (UKAS) is the participation in PT schemes with those providers that have been accredited to ISO/IEC 17043:2010. This International Standard specifies criteria and the general requirements for the competence of the PT providers and their responsibility for all tasks in the development and operation of the PT scheme. Some of the main differences introduced with ISO 17043 are summarized in Table 2.
Assessment of conformance
When conducting an assessment of a PT scheme for conformance to ISO 17043:2010, the assessors will take a holistic approach looking at the management system as a whole. The assessment will include areas such as scheme organization, scheme management, evaluation processes, technical competence and impartiality and integrity. Each separate area of the PT scheme are all interlinked and therefore when accreditation is granted by the accreditation body (UKAS in the UK) it will not be given on a single fact but the overall competence of the PT provider. Accreditation to ISO 17043:2010 can be a hard and thorough task for PT providers to undertake but once accreditation is granted it provides the necessary assurance of a competent and professional scheme which can provide an open and honest service whilst maintaining confidentiality for all those participants enrolled in the scheme.
Summary
The 2013 Barnes review into quality assurance in the NHS pathology services reinforced the importance of quality assurance and this article has discussed the implications of recently introduced ISO standards for clinical pathology departments (ISO 15189:2012) as well as for EQA scheme providers (ISO 17043:2010). This strengthens and standardizes the systems used in clinical testing laboratories and ensures high quality and comparable results for patient tests.
References
1. James D, Ames D, Lopez B, Still R, Simpson W, Twomey. External quality assessment: best practice. J Clin Pathol. 2014; doi: 10.1136/jclinpath-2013-20621.
2. Bullock DG. External quality assessment schemes for clinical chemistry in the United Kingdom. Ann Ist Super Sanita 1995; 31: 61–69.
3. Barnes I. Pathology Quality Assurance Review 2014. www.england.nhs.uk/wp-content/uploads/2014/01/path-qa-review.pdf
4. Thompson M, Ellison SLR, Wood R. The International Harmonized Protocol for the proficiency testing of analytical chemistry laboratories (IUPAC Technical Report). Pure Appl Chem. 2006; 78: 145–196.
5. Arnaud J, Weber J-P, Weykamp CW, Parsons PJ, Angerer J, Mairiaux E, Mazarrasa O, Valkonen S, Meditto A, Patriarca M, Taylor A. Quality specifications for the determination of copper, zinc, and selenium in human serum or plasma: evaluation of an approach based on biological and analytical variation. Clin Chem. 2008; 54(11): 1892-1899.
6. Summary of ISO 15189 additional requirements. CPA UK Ltd, 2012. http://www.ukas.com/Library/Services/CPA/Summary%20of%20Idifferences%20betwen%20ISO%2015189%20&%20CPA.pdf
The authors
Sarah-Jane Bainbridge and Chris F. Harrington* PhD
TEQAS, Trace Element Centre, Surrey Research Park, Guildford GU2 7YD, UK
*Corresponding author
E-mail: Chris.harrington1@nhs.net