Fibroblast growth factor-23: an emerging biomarker in chronic kidney disease
Fibroblast growth factor-23, a key regulator of phosphate and 1,25-dihydroxyvitamin D metabolism, appears to be an independent risk factor for mortality among chronic kidney disease patients. However, sample stability and poor analytical agreement between detection methods still need to be addressed for it to become a reliable biomarker.
by Dr A. Kumar, Dr W. Herrington, Dr S. Clark and Dr M. Hill
The function of fibroblast growth factor-23 and its role in chronic kidney disease
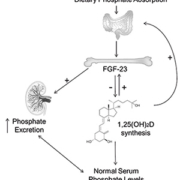
Fibroblast growth factor-23 (FGF-23) was identified as the key regulator of phosphate homeostasis from a study of renal phosphate-wasting condition autosomal dominant hypophosphatemic rickets [1]. It is secreted principally by bone-forming osteocytes and osteoblasts in response to increased dietary phosphate intake and abnormally elevated serum phosphate concentration (hyperphosphatemia). FGF-23 acts to correct raised phosphate levels by increasing urinary phosphate excretion, by direct inhibition of renal tubular phosphate reabsorption, and via reducing dietary phosphate absorption, by suppressing 1α-hydroxylation of 25-dihydroxyvitamin D to form active 1,25-dihydroxyvitamin D [1,25(OH)2D]. Low 1,25(OH)2D production also provides a negative feedback signal in phosphate homeostasis by inhibiting further FGF-23 secretion (Fig.1)[2, 3].
Chronic kidney disease (CKD) commonly causes a fall in glomerular filtration rate resulting in a reduced capacity for phosphate excretion [4]. FGF-23 levels increase early in CKD, often before any detectable rise in phosphate concentration [5] and those with the severest form of CKD, end-stage renal disease (ESRD), have FGF-23 levels that are 100 to 10,000-fold higher than healthy controls [4, 6]. Sustained suppression of renal 1,25(OH)2D synthesis by high FGF-23 levels contributes to lower serum calcium concentration, a stimulant of parathyroid hormone (PTH) secretion. PTH maintains normal serum calcium concentration by promoting reabsorption of the calcium from its reservoir in bone. The abnormal elevated levels of FGF-23 seen in CKD thus results in a disruption of the homeostatic balance of calcium and phosphate and this may impact on many normal processes including bone mineral metabolism and cardiovascular function. The consequences of prolonged derangement of bone mineral metabolism (known as CKD-mineral bone disease; CKD-MBD) is bone pain and increased fracture risk. CKD-MBD may also accelerate calcification of the vascular tree, a process that may explain some of the significantly increased cardiovascular risk in those with CKD [7]. Indeed, among CKD patients, several studies have shown FGF-23 to be an independent risk factor for mortality [8] and among those not on dialysis it appears to also predict CKD progression [4, 9]. Treatments that might positively impact on FGF-23 levels, for example, reducing dietary phosphate absorption with phosphate binders may therefore have beneficial effects on bone, renal and cardiovascular outcomes in those with CKD (both in those with hyperphosphatemia and those with high-normal serum phosphate concentration).
Methods for FGF-23 assessment
In vitro studies have shown that some of the FGF-23 synthesized by the osteocytes is cleaved between amino acid 179 and 180 by furin (a type I precursor convertase) releasing a C terminal fragment. Current immunometric methods detect either ‘intact’ FGF-23 (iFGF-23, ~32 KDa) or ‘C-terminal’ fragments (cFGF-23, ~14 KDa) in plasma or serum. The cFGF-23 assays recognize two epitopes in the C-terminus, thereby recognizing both iFGF-23 and cFGF-23 fragments. The intact assays recognize only the iFGF-23 because the epitopes flank the cleavage site [2]. At present, there is no reference method, or consensus to indicate which assay type is the most suitable for measuring circulating FGF-23. If all circulating FGF-23 is intact and biologically stable, concentrations detected by the intact and C-terminal assays should be comparable [10]. However, there is a paucity of data confirming this and so caution should be used when comparing studies using the different methods.
Several studies have assessed the performance of commercially available enzyme-linked immunosorbent assays (ELISAs) for FGF-23. Heijboer and co-workers evaluated the performance of one cFGF-23 assay (Immutopics, USA) and two iFGF-23 assays (Immutopics and Kainos Laboratories Inc., Japan) using samples from healthy volunteers and patients with expected high levels of FGF-23 [11]. Intra- and inter-assay variations were assessed in approximately 100 samples with low, normal and high FGF-23 concentrations providing <20% CV for the cFGF-23 Immutopics and iFGF-23 Kainos assays. A high intra-assay variation (22–61%) was observed for the Immutopic intact assay which may be due to lot-to-lot variation [11]. A potential difficulty observed with the Kainos intact assay is poor assay performance when using an automated plate washer, as directed in their protocol. Heijboer and co-workers found acceptable results were only obtained when the wells were washed manually, which made this method impractical for measurement of large numbers of samples [11]. However, a later version of the Kainos assay protocol (from October 2010 onwards) includes an improved wash instruction. Using plasma samples from patients with renal impairment, Devaraj and co-workers also found good inter- and intra assay precision for cFGF-23 assay (CV between 4–10.5%), however, the CVs for iFGF-23 Immutopic assay were found to be poor (6–37.5%) [12].
Poor analytical agreement exists between the commercially available FGF-23 assays, due principally to the lack of a reference method. The performance of four commercially available methods [iFGF-23 assays from Immutopics, Kainos and Millipore (USA) and a cFGF-23 assay from Immutopics] were recently compared using plasma from 31 healthy adults and 36 patients undergoing hemodialysis [13]. A broad range of FGF-23 values were obtained: whereas the patient ranges fell between 154–2561 pg/mL and 447–2063 pg/mL for iFGF-23 and cFGF-23 assay respectively, the levels for healthy adults ranged from 9.9–62 pg/mL for the two assay types. Poor analytical agreement was observed between the assays particularly in the patient group. No agreement of test results was found between the iFGF-23 and cFGF-23 assays and this was more evident at physiological concentrations than in the haemodialysis group [13]. The lack of analytical agreement between these commercially available FGF-23 methods emphasizes that they cannot be used interchangeably and that a comparison of findings from different assays requires careful interpretation. The above evaluation study was performed with plasma samples [13]. A further consideration is some assays are restricted to the sample type that can be used. The iFGF-23 Kainos assay is suitable for both plasma and serum; however, the cFGF-23 Immutopics assay is established only for plasma [10, 13] providing lower or undetectable results in serum [10, 12]. Further method comparisons, ideally on larger numbers of samples and in different patient groups, would provide a valuable insight in this area and help identify which assay type is the most suitable for measuring circulating FGF-23. Nevertheless, studies measuring either intact or C-terminal FGF-23 have reported associations with mortality risk and decline in renal function [4, 14].
Stability of FGF-23: implication for large scale epidemiological studies
Limited evidence exists for the short-term stability of FGF-23 in collected blood samples (6 hours or less) and no information is available for its long-term stability in stored samples. Smith and co-workers investigated the short-term pre-analytical stability of FGF-23, measured using iFGF-23 and cFGF-23 ELISAs from Immutopics, by performing a number of timed experiments with blood taken from 15 patients with mild CKD [6]. The effect of aprotinin, a serine protease inhibitor, and a commercially available protease inhibitor cocktail to preserve FGF-23 after blood collection was also investigated [6]. In the absence of any preservative or inhibitor, iFGF-23 degraded by approximately 40% within 2 hours of collection even when the blood samples were separated into plasma. Conversely, 2 hours after blood collection the FGF-23 concentrations had increased by approximately 35% using the cFGF-23 assay. However, with the addition of the protease inhibitor cocktail the stability of both iFGF-23 and cFGF-23 in the samples extended up to 4 hours (less than 10% change). Based on this evidence, it appears that FGF-23 cannot be measured reliably in blood samples collected without the use of any preservative or inhibitors. This could be a serious limitation for large-scale epidemiological studies, particularly if samples have already been collected and are in storage and for blood collection methods that need to be simple in order to be cost-effective and feasible.
Preliminary findings from our laboratory using samples from 54 CKD patients suggest that FGF-23 (measured by both the iFGF-23 Kainos ELISA and the cFGF-23 Immutopics ELISA) remains stable in whole blood stored for up to 96 hours without the use of a preservative [15]. The apparent lack of agreement with the results from Smith et al. may be explained by differences in the methods of sample collection. Smith et al. used a single K2-EDTA blood tube for each participant which was re-sampled at each time-point whereas we collected separate blood tubes corresponding to each time-point.
Large-scale epidemiological studies often involve the long-term frozen storage of samples prior to biomarker analyses, particularly nested case-control designed studies where it may take several years for sufficient incident cases to materialize. Limited information is available to help understand the impact of long-term frozen storage on the stability of many biomarkers, including FGF-23. Studies investigating the stability of biomarkers in different sample types stored at various temperatures (for example, −40 °C, −80 °C and in liquid nitrogen vapour) will have immense value, particularly in support of long-term blood based prospective studies and biobanks.
Summary
FGF-23 is a key regulator of phosphate homeostasis and has emerged as an important biomarker in patients with CKD. Despite an increasing amount of literature, there are still unanswered questions related to FGF-23 sample stability and the availability of robust reliable methods for measuring FGF-23. Further studies into these areas will improve the quality of clinical research into the use of FGF-23 as a potential early biomarker in CKD.
References
1. ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000; 26: 345–348.
2. Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol. 2010; 21: 1427–1435.
3. Quarles LD. Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease. Exp Cell Res. 2012; 318: 1040–1048.
4. Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011; 305: 2432–2439.
5. Russo D, Battaglia Y. Clinical significance of FGF-23 in patients with CKD. Int J Nephrol. 2011; 2011: 364890.
6. Smith ER, Ford ML, Tomlinson LA, Weaving G, Rocks BF, Rajkumar C, Holt SG. Instability of fibroblast growth factor-23 (FGF-23): implications for clinical studies. Clin Chim Acta 2011; 412: 1008–1011.
7. London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003; 18: 1731–1740.
8. Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009; 24: 2792–2796.
9. Titan SM, Zatz R, Graciolli FG, dos Reis LM, Barros RT, Jorgetti V, Moyses RM. FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clin J Am Soc Nephrol. 2011; 6: 241–247.
10. Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, Wolf M, Salusky IB, Jüppner H. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010; 95: 578–585.
11. Heijboer AC, Levitus M, Vervloet MG, Lips P, ter Wee, PM, Dijstelbloem HM, Blankenstein MA. Determination of fibroblast growth factor 23. Ann Clin Biochem. 2009; 46: 338–340.
12. Devaraj S, Duncan-Staley C, Jialal I. Evaluation of a method for fibroblast growth factor-23: a novel biomarker of adverse outcomes in patients with renal disease. Met Syndr Relat Disord. 2010; 8: 477–482.
13. Smith ER, McMahon LP, Holt SG. Method-specific differences in plasma fibroblast growth factor 23 measurement using four commercial ELISAs. Clin Chem Lab Med. 2013; 51: 1971–1981.
14. Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, Konig P, Kraatz G, Mann JF, Muller GA, Kohler H, Riegler P. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007; 18: 2600–2608.
15. Illingworth N, Edmans M, Clark S, Kumar A, Sutherland S, Herrington W, Hill M. Investigation of FGF-23 sample & assay suitability for large scale epidemiological studies. Ann Clin Biochem. 2013; 50: 73–74
The authors
Aishwarya Kumar1* PhD; Will Herrington2 MBBS, MRCP; Sarah Clark1 PhD; and Michael Hill1 PhD
1Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), University of Oxford, Oxford, UK
2Oxford Kidney Unit, Oxford University Hospitals, Oxford, UK
*Corresponding author
E-mail: Aishwarya.Kumar@ctsu.ox.ac.uk