Granulomatous infections in the bone marrow
Granulomas are a rather uncommon, yet diagnostically helpful finding in trephine bone marrow biopsies. Being indicative for a rather limited number of various underlying diseases (ranging from infections to autoimmunopathies and malignant tumours), the finding of granulomas in the bone marrow should precipitate further analyses to uncover the underlying condition.
by Dr Thomas Menter and Dr Alexandar Tzankov
Microscopic aggregations of epithelioid histiocytes are referred to as granulomas and the respective inflammatory process is called granulomatous. Granulomas are a well-known feature in pathology, with the first description ranging back to the 17th century. They show variable morphologic features including central areas of necrosis (necrotizing granulomas) or suppuration (microabsceding or suppurative granulomas), incorporation of foreign material or presence of giant cells due to the fusion of macrophages. Granuloma formation is usually associated with local CD4+ T-cell activation (and numeric increase of CD4+ T-cells at the site of granuloma formation, thus ‘consuming’ CD4+ T-cells and leading to skewing of the CD4/CD8 ratio in the peripheral blood in favour of CD8) and production of interleukin 2 and 12, Interferon (IFN) γ, and tumour necrosis factor α (TNF-α) [1, 2]. In general, granulomas are provoked by agents that are difficult to eradicate by the enzymes of histiocytes, for example because of the different phospholipid composition of the former (e.g. mycolic acids) or because of the specific enzymo/immunogenetic background of the host. The number of causative agents in granulomatous responses is rather smaller than in other inflammatory patterns. Therefore granulomas are considered to be ‘specific’; narrowing down the possible causative factors, their identification and subsequent search for possible underlying agents and conditions supports the establishment of more precise clinicopathological diagnoses.
Morphology of bone marrow granulomas and etiologic considerations
Granulomatous processes involving the bone marrow (BM) can be divided into three subgroups based on morphology and clinicopathological context: (1) lipogranulomas, (2) infectious epithelioid granulomas, and (3) epithelioid granulomas associated with immune dysregulation. Importantly, most inborn immunodeficiency disorders are accompanied by increased granuloma formation [3]. Examples of these disorders include Blau syndrome, CVID (common variable immune deficiency), RAG (recombination-activating genes) deficiency, XIAP (X-linked inhibitor of apoptosis) deficiency and chronic granulomatous disease. Depending on whether solely the BM or other organs are also involved, granulomatous BM processes may represent isolated findings or reflect the involvement of a systemic disorder. It is obvious that detection of granulomatous BM processes should be followed by an integrative diagnostic work-up considering the clinical history (travel and drug history) and presentation, but also applying imaging techniques and molecular detection methods including serology, in situ uncover techniques and PCR- and sequencing-based procedures.
Lipogranulomas
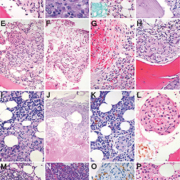
Lipogranulomas are found in up to 10% of BM samples. They are not thought to be significant since probably not linked to specific underlying disorders. Yet, they may be more commonly observed in patients with acute febrile illnesses. They consist of aggregates of histiocytes with variably sized lipid vacuoles (Fig. 1A), which tend to gradually disappear with time resulting in a morphological appearance of lipogranulomas indistinguishable from epithelioid granulomas. When detecting BM lipogranulomas, special attention must be given to the periodic acid Schiff (PAS)/diastase-PAS stains so as not to miss involvement by Whipple’s disease, in which the foamy histiocytes stain positively (Fig. 1B); in suspect cases, the diagnosis of Whipple’s disease can be enhanced by additional Ziehl–Neelsen and Warthin–Starry stains as well as by PCR- and sequencing-based procedures. Granulomas in Erdheim–Chester disease might sometimes resemble lipogranulomas [4].
Epithelioid granulomas
Epithelioid granulomas are found in <1% of BM samples [5]. They are more frequent in certain geographic areas and in samples from patients with immune dysregulation. They are considered significant as they are associated with various infectious (Fig. 1C), immune dysregulatory (Fig. 1D) and neoplastic disorders (Fig. 1E). After clinicopathological and molecular work-up, a specific etiology of BM epithelioid granulomas can be attributed in up to 80% of cases. Such granulomas consist of loose [particularly in severely immunocompromised patients (the lower the CD4+ counts or the membrane-bound TNF-α and the more virulent the infectious agent, the looser the granuloma, Fig. 1F)] to cohesive clusters of epithelioid histiocytes with accompanying lymphocytes, eosinophilic (Fig. 1G) and neutrophilic granulocytes, and giant cells (Fig. 1H). In patients with infectious diseases, these granulomas mostly contain organisms, which should be actively sought for and if possible visualized (e.g. mycobacteria, histoplasmata, Bartonella henselae, treponemata, Leishmania spp., toxoplasmata) using special stains such as PAS, Ziehl–Neelsen, Fite, Grocott, May–Grünwald–Giemsa, Warthin–Starry, etc. Immunohistochemistry (Fig. 1L, insert) or molecular genetic methods might be necessary for etiologic assignment. A particular CD8 predominance in the BM interstitium often accompanies virus infections [e.g. cytomegalovirus (CMV) and Epstein–Barr virus (EBV)] and might serve as an additional diagnostic hint [6].
Different granuloma morphotypes
There are different granuloma morphotypes, which should be recognized because they may give a clue to the underlying disorder [7]. Caseating granulomas (i.e. granulomas with central necrosis) are usually caused by infectious agents such as mycobacteria, histoplasmata, Francisella tularensis, Yersinia pestis or brucellaceae. Ring-form granulomas (Fig. 1I) can be observed in acute virus infections (e.g. CMV), brucellosis, leishmaniasis and those appearing as ‘doughnut rings’ in Q-fever. Foreign body granulomas are rarely seen, but can be encountered in patients after repeated BM sampling (e.g. containing displaced keratin), or in patients with degenerative and debilitating disorders, in whom subchondral epithelioid clusters may raise differential diagnostic concerns of metastatic carcinomas (Fig. 1J).
Occasionally detached giant osteoclasts in patients receiving long-term bisphosphonate therapy may be conventionally indistinguishable from foreign body giant cells, but immunohistochemistry for tartrate-resistant acid phosphatase (TRAP) can be helpful, as osteoclasts are intensively positive. Sarcoid-type granulomas (Fig. 1K) consist of compact epithelioid collections and are less specific as they can accompany genuine sarcoidosis and autoimmune disorders such as, for example, rheumatoid arthritis [8].
The role of giant cells in granulomas
Another clue to the etiology in a granulomatous inflammation might be the type of giant cells present around the epithelioid histiocytes [9]. Langhans giant cells are characteristically seen in tuberculosis, and appear with nuclei arranged in a horseshoe-like fashion at the periphery of the cell below the cell membrane. In contrast, in Touton giant cells that are typically observable in areas of fat necrosis, the nuclei form a complete ring, and the cytoplasm is rather foamy than eosinophilic. In foreign body giant cells, the nuclei do not show a particular order but are rather haphazardly distributed. Besides, the foreign material incorporated in these cells (a clue to this diagnosis) is often visible by conventional or polarized light, being either pigmented or birefringent.
A particular vascular association of granulomas should raise suspicion of vasculitis, either primary like granulomatous polyangiitis (formerly known as Wegener’s disease), or secondary/infectious such as syphilis (Fig. 1L).
Granulomas and malignancies
Importantly, detection of BM granulomas does not exclude an underlying malignant process; on the contrary, BM granulomas may accompany various lymphoproliferative processes such as Castleman’s disease (Fig. 1M), B- and T-cell (so called ‘non-Hodgkin’) as well as Hodgkin lymphomas (HL) [10, 11], but also the BM spread of solid tumours such as lobular breast cancer [12]. There is a significant association between BM granulomas and non-Hodgkin lymphoma spread to the BM. Granulomas due to IFN therapy can be encountered in lymphoma patients [especially patients suffering from splenic marginal zone B-cell lymphoma and diffuse large B-cell lymphoma (DLBCL)] treated for underlying chronic hepatitis B- or C-virus (HBV, HCV) infections – both viruses are known to increase the risk of these lymphomas (Fig. 1N). Occasionally, granulomatous reactions might obscure lymphoma. This may particularly apply to HL, in which on the one hand granulomatous reactions can occur independently of BM infiltration by HL (not worsening patients’ prognosis; indeed 5% of HL patients have BM granulomas without BM involvement by lymphoma), and on the other hand BM involvement by HL is usually granulomatous with only a handful Reed–Sternberg cells. Therefore step-sections supported by immunohistochemistry (CD15, CD30) are warranted if BM granulomas are encountered in patients suffering from HL (Fig. 1O). Patients with lymphomas are at an increased risk of developing infections, which may also lead to granulomas and should therefore raise awareness for the differential work-up for infectious agents as described above. Finally, patients suffering from sarcoidosis are at increased risk of lymphoma (odds ratio 2) and incipient lymphomas [especially Burkitt lymphomas, DLBCL, small lymphocytic B-cell lymphomas, lymphoplasmacytic lymphomas and peripheral T-cell lymphomas (PTCL)] may provoke sarcoid-like reactions summarized in the so called ‘lymphoma-sarcoidosis syndrome’. To be comprehensive, apart from IFN, several other treatment compounds such as hematopoietic growth factors like G-CSF (Fig. 1K), TNF-α blockers, BCG vaccination, allopurinol, amiodarone, antipsychotics, phenytoin, sulfonamides, etc., can lead to BM granuloma formation [13].
Further etiologies of granulomas in bone marrow biopsies
Several other neoplastic and non-neoplastic conditions [like systemic mastocytosis, (Langerhans cell) histiocytoses], genuine histiocytic and metabolic/storage disorders (like Erdheim–Chester, Rosai–Dorfman and Gaucher’s disease), sea blue histiocytoses, hemophagocytic lympho-histiocytosis, T-cell and histiocyte-rich B-cell lymphomas or lymphomatoid granulomatosis (Fig. 1P) involving the BM can morphologically mimic granulomas and should be distinguished from the latter by means of ancillary studies.
Technical considerations
A comprehensive review on technical handling of BM biopsies to obtain optimal immunohistochemical results has been published recently [14]. Trephine BM biopsies are best taken from the iliac crest. For further analysis, they should be sent to the pathology institutions in 10% buffered formalin (final formaldehyde concentration 4%) in order to prevent autolytic changes and to allow for an optimally preserved morphology. For decalcification, chelate binders such as EDTA should be used. We discourage the use of other decalcificative agents such as formic acid or mercury containing agents such as SUSA-fixation because of the alteration of proteins and destruction of DNA as well as for health issues for the laboratory staff with regard to mercury exposure. Standard special stains of BM biopsies include H&E, PAS, Giemsa and Gömöri stains. Applying the PAS and the Gömöri stain can highlight fungi, but in the case of artefacts caused by BM fibrosis, other than Gömöri silver, stains such as a Grocott stain are helpful. The Giemsa stain is useful in the context of leishmaniasis and toxoplasmosis. Other histochemical stains used in the context of infectious diseases include Fite or Ziehl–Neelsen stains (for less or more acid-fast bacteria) and the Warthin–Starry stain. Besides which, many infectious agents including parasites, bacteria and viruses can be detected using immunohistochemistry or in situ hybridization methods. In the context of lymphoma or suspicion of carcinoma, additional immunohistochemical stains are recommended including CD3, CD5, CD15, CD20, CD30 and pan-cytokeratin.
Take home messages
1. 0.5% of BM biopsies display epithelioid granulomas, up to 10% lipogranulomas.
2. 80% of such patients with epithelioid granulomas are symptomatic and in 80% of them a specific integrative diagnosis is possible, mostly infectious diseases (30–50%), achievable by applying:
• special stains
• field studies (travelling, ethnicity, clinical exam, drug exposure)
• serology
• PCR-based molecular genetic studies.
3. Detection of BM granulomas does not exclude an underlying malignant process, in fact this possibility must be actively sought for and, if needed, excluded.
4. Genuine neoplastic and non-neoplastic histiocytic disorders represent important differential diagnoses to granulomatous BM process.
References
1. Zumla A, James DG. Clin Infect Dis. 1996; 23(1): 146–158.
2. Helming L, Gordon S. Trends Cell Biol. 2009; 19(10): 514–522.
3. Rosé CD, Pans S, Casteels I, et al. Rheumatology 2014; 54: 1008–1016.
4. Kim NR, Ko YH, Choe YH, et al. Int J Surg Pathol. 2001; 9(1): 73–79.
5. Brackers de Hugo L, Ffrench M, Broussolle C, et al. Eur J Intern Med. 2013; 24(5): 468–473.
6. Blanco P, Viallard JF, Parrens M, et al. Lancet 2003; 362: 1224.
7. Eid A, Carion W, Nystrom JS. West J Med. 1996; 164(6): 510–515.
8. Rao DA, Dellaripa PF. Rheum Dis Clin North Am. 2013; 39(2): 277–297.
9. Brodbeck WG, Anderson JM. Curr Opin Hematol. 2009; 16(1): 53–57.
10. Brunner A, Kantner J, Tzankov A. J Clin Pathol. 2005; 58(8): 815–819.
11. Brunning RD, McKenna RW. Pathol Annu. 1979; 14 Pt 1: 1–59.
12. Kettle P, Allen DC. J Clin Pathol. 1997; 50(2): 166–168.
13. Bhargava V, Farhi DC. Hematol Pathol. 1988; 2(1): 43–50.
14. Torlakovic EE, Brynes RK, Hyjek E, et al. Int J Lab Hematol. 2015; 37: 431–449.
The authors
Thomas Menter MD, Alexandar Tzankov* MD
Institute of Pathology, Basel, Switzerland
*Corresponding author
E-mail: alexandar.tzankov@usb.ch
Acknowledgment
This article is based on the presentation ‘Granulomatous infection (and reactions) in the bone marrow other than mycobacteria’ by Dr Tzankov at the 26th European Congress of Pathology in London, UK, 2014.