H. pylori infection: a laboratory perspective
Infection with Helicobacter pylori is indicated in disorders of the upper gastrointestinal tract, from dyspepsia and ulcer disease to gastric carcinoma. It can be detected during endoscopy or non-invasively using breath, stool or blood samples, each of which has advantages and limitations depending on patient and population circumstances.
by Sarah Knowles and Julia M. Forsyth
Helicobacter pylori
Helicobacter pylori is probably the most common cause of bacterial infection in humans, present in up to 50% of the world’s population [1]. The presence of such a microorganism in the stomach was first reported almost 100 years ago [2] but was not taken seriously until Marshall and Warren demonstrated a strong association between the presence of an unidentified curved bacillus and inflammation on a gastric biopsy [3]. The organism was initially placed in the Campylobacter genus but as further morphological, structural and genetic information was determined a new genus called Helicobacter was created. After identification, Marshall demonstrated the role of H. pylori in antral gastritis by self-administration of the bacteria and also showed that it could be cleared by the use of antibiotics and bismuth salts.
H. pylori and disease
Infection with H. pylori is known to be a contributing factor in producing gastritis [4]. Studies have shown that infection with H. pylori leads to increased release of gastrin by the antral mucosa (through a mechanism that is as yet undefined), which resolves on eradication [5, 6]. There is a high prevalence of H. pylori positive chronic gastritis in patients with duodenal and gastric ulcers (70% and 90% respectively) [6, 7]. These ulcer patients have been shown to have an exaggerated response to the increased gastrin, even compared to asymptomatic H. pylori-positive patients [6], leading to excess acid production and a deterioration from the initial inflammation of gastritis to mucus layer erosion by peptic acid. H. pylori is also linked with gastric carcinomas and mucosa-associated lymphoid tissue (MALT) lymphomas with one study showing that 62% of patients with low-grade gastric MALT lymphoma entering complete remission 12 months after H. pylori eradication therapy [8]. The vast majority of individuals with H. pylori infection, however, do not have ulcerative disease, but are symptomless carriers [4].
The survival capabilities of H. pylori in the stomach make it difficult to eradicate. Effective treatment requires a multidrug ‘triple therapy’ regime consisting of two antibiotics from clarithromycin, metronidazole, amoxicillin and tetracycline combined with an acid suppressant or bismuth compounds [9], and in areas of high clarithromycin resistance, quadruple therapy including two antibiotics, proton pump inhibitors (PPIs) and bismuth [10].
Testing for H. pylori
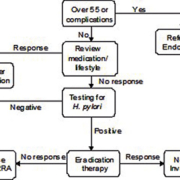
The definitive identification of H. pylori is by histological examination of biopsies stained with Giesma stain or Warthin–Starry silver impregnation [4]. Other biopsy immunohistochemical techniques using specific antibodies against H. pylori are also available along with rapid biopsy urease tests (otherwise known as the CLO-test), where a biopsy specimen is placed in a urea broth containing a pH indicator. Testing by any of these methods requires endoscopy which, apart from being very unpleasant for the patient, carries risk and is very costly, and is therefore unsuitable for routine screening for H. pylori in patients with a low risk of cancer. In these cases a ‘test and treat’ strategy is recommended using non-invasive tests (Fig. 1) [10].
There are three common non-invasive tests available for the routine detection of H. pylori:
1. The urea breath test (UBT)
2. Stool test for H. pylori antigen
3. Serum test for H. pylori antibody.
Urea breath test
The UBT is generally considered to be the ‘gold standard’ non-invasive test and is recommended as the first-choice screening test both in the diagnosis of infection and post-eradication testing [9–11].
Urea labelled with 13C is taken as a drink or a capsule and urease produced by H. pylori acts upon the urea to produce 13C-labelled carbon dioxide. This is absorbed into the blood stream and exhaled in the breath. The ratio of 13CO2 : 12CO2 is measured using an isotope ratio mass spectrometer (IRMS), and the ratios before and after the administration of urea are compared to give the result.
Apart from being the ‘gold standard’ test the other main advantage of the UBT is the stability of the breath samples once collected. This allows storage without refrigeration and transportation to the central referral laboratory for analysis. In the UK this permits sample collection at local health centres rather than hospital settings, which is convenient for patients. Worldwide this can extend to large, remote areas with limited facilities or courier transport links to laboratories, for example rural Africa and Australia, permitting testing in all communities. Local studies have also shown the test to be very popular with patients as sample collection is simple and pain free [12].
A disadvantage of the UBT is that PPIs and antibiotics need to be withheld for 2 and 4 weeks respectively before testing to avoid potential false-negative results as a consequence of decreased bacterial load [10]. PPIs particularly are commonly prescribed to alleviate the symptoms of dyspepsia and patients can be reluctant to withhold such medication pending tests. As with all testing cost is also an important issue. IRMS is a specialized laboratory technique requiring expensive equipment and highly trained laboratory personnel. These fixed costs can make the UBT an expensive option but running costs and reagents for such equipment are relatively low, and therefore centralized high workload laboratories can provide a very cost-effective service [12].
Stool antigen test
The stool antigen test uses enzyme-linked immunosorbent assay (ELISA) methodology to detect H. pylori antigen. ELISA technology is available in many laboratories, thus making the test easy to implement at local laboratories. It is also reported to be more cost effective than the UBT [13], although local method validation of commercially available ELISA kits (for a workload of 3500 tests per year) showed the costs to be almost identical when samples were analysed in duplicate [12]. Duplicate analysis was recommended due to poor replicate precision, probably as a result of the heterogeneity of stool in the samples. As workload increases, e.g. at centralized laboratories, the UBT actually becomes the cheaper alternative because of the low running/reagent costs [12]. A final advantage of the stool antigen test is that there is no need to attend a clinic or phlebotomy appointment for sample collection. The patient can collect the sample at home and deliver it to their GP/local courier collection or laboratory at their convenience.
Current UK guidelines for the investigations do not recommend the stool antigen test in the post-eradication setting [9]. There is, however, no discrimination between polyclonal and monoclonal assays in these guidelines. Initial commercial ELISA assays were based upon polyclonal antibodies which showed reasonable performance in untreated patients with reported sensitivities of 91–93% and likewise specificities of 91–93% [14–16]. However, in the post-eradication setting results are less convincing with reported sensitivities ranging from 67–89% [14–19]. The introduction of monoclonal antibody assays has lead to an improvement in the diagnostic accuracy with reported sensitivities of 94–98% in both untreated adults and children [16, 20, 21], and pooled specificity of 97% [22]. Several more-recent studies using monoclonal antibody kits with favourable results have been published since the guidelines were drawn up with pooled sensitivities and specificities of 93% and 96% respectively [22]. This is reflected in the recent European study group findings which have recently been updated to include the monoclonal ELISA test as accurate in the post eradication setting, suitable for use if the UBT is not available [10], and local studies have validated the stool test as a suitable alternative to the UBT both in diagnosis and post-eradication settings [12].
The stool antigen test offers no further advantage to the UBT with regard to patient preparation as antibiotics and PPI medication should also be withheld before testing and locally the test is unpopular with patients who do not wish to provide stool specimens. Stability is also a disadvantage as specimens need to be refrigerated, and if not tested immediately, frozen within 48 hours [23]. Samples therefore need to reach the laboratory quickly or be transported frozen, providing logistical challenges in more remote locations.
Rapid point-of-care testing or ‘office’ stool antigen assays are available but have limited accuracy and are not recommended by current guidelines [10, 11].
Serum antibody test
The serum antibody test is an ELISA assay for IgG H. pylori antibodies, which again has the advantage of being a standard laboratory technique. It is also the cheapest testing option as reagent costs are low compared to the stool antigen test. Unfortunately this test has been shown to have poor diagnostic accuracy [11] especially in the post-eradication setting. This is thought to be due to the fact that the antibody levels persist in the blood for a long time and therefore lead to false-positive results after treatment. It may, however, have a place in the diagnosis of infection setting on occasions where PPI or antibiotic medications cannot be withheld, or for testing young children for whom the UBT is inappropriate and the likelihood of previous infection is low. Point-of-care or office-based serology tests are available, although their performance has been shown to be inadequate and they are not recommended for use in testing for H. pylori [10, 11].
Conclusion
In conclusion, H. pylori detection and eradication can lead to significant health benefits to the world’s population, not only alleviating life-restricting symptoms but also preventing the development of more serious disease. Several reliable methods of laboratory testing are now available, the choice of which depends on the patient population, facilities available, workload and networking possibilities and pre-existing medical conditions.
References
1. Cover TL, Blaser MJ. Adv Int Med. 1996; 41; 85–117.
2. Maden E, et al. Am J Clin Pathol. 1988; 90: 450–453.
3. Marshall BJ, Warren JR. Lancet 1984; 1(8390): 1311–1315.
4. Mera SL. Br J Biomed Sci. 1995; 52; 271–281.
5. Smith JTL, et al. Gut 1990; 31: 522–525.
6. El-Omar E, et al. Gut 1993; 34: 1060–1065.
7. Parsonner J, et al. N Engl J Med. 1991; 325: 1127–1131.
8. Fischbach W, et al. Gut 2004; 53: 34–37.
9. NICE. Dyspepsia. Management of dyspepsia in adults in primary care. Clinical Guideline 17 2004; http://www.nice.org.uk/nicemedia/pdf/CG017NICEguideline.pdf.
10. Malfertheiner P, et al. Gut 2012; 61: 646–664.
11. Malfertheiner P, et al. Gut 2007; 56: 772–781.
12. Research Studies, Pathology Department, Royal Derby Hospital. Data awaiting publication.
13. Elwyn G, et al. Br J Gen Pract. 2007; 57: 401–403.
14. Roth DE, et al. Clin Diagn Lab Immunol. 2001; 8: 718–723.
15. Gisbert JP, et al. Am J Gastroenterol. 2001; 96: 2829–2838.
16. Gisbert JP, et al. Helicobacter 2004; 9: 347–368.
17. Perri F, et al. Am J Gastroenterol. 2002; 97: 2756–2762.
18. Bilardi C, et al. Aliment Pharmacol Ther. 2002; 16: 1733–1738.
19. Erzin Y, et al. J Med Microbiol. 2005; 54: 863–866.
20. Koletzko S, et al. Gut 2003; 52: 804–806.
21. Weingart V, et al. J Clin Microbiol. 2004; 42: 1319–1321.
22. Gisbert JP, et al. Am J Gastroenterol 2006; 101: 1921–1930.
23. Oxoid Amplified IDEIA HPStAR Kit insert Ref K6630.
The author
Sarah Knowles MSc, FRCPath and Julia Forsyth MSc, FRCPath
Pathology Department, Royal Derby Hospital, Derby, UK
*Corresponding author
E-mail: Sarah.knowles@nhs.net