Improved productivity and workflow efficiencies enhance viral load service provision
A series of independent evaluations of the Beckman Coulter DxN VERIS Molecular Diagnostics System demonstrated how workflow efficiencies that can be achieved with the DxN VERIS have the potential to improve productivity, while making the best use of existing space and staffing levels.
Background
Virology laboratories throughout the world face a number of challenges that need to be addressed in order to meet service user requirements. These include:
- Expanding molecular diagnostic workloads
Include an increase in requests for viral load assays for targets, such as human immunodeficiency virus type 1 (HIV-1), hepatitis C virus (HCV), hepatitis B virus (HBV) and cytomegalovirus (CMV). This growth may be attributed, in part, to the development of new therapeutic strategies or the move away from other traditional “non-molecular” methods. - Limited space and workflowinefficiencies
Many laboratories have to cope with these expanding workloads in already crowded working environments.
Many existing methods used for obtaining HIV-1, HCV, HBV and CMV viral loads involve multiple platforms and are thus using valuable laboratory space.
Evaluation of a new automated molecular diagnostics method
In 2014/2015, a number of hospital laboratories across Europe became beta trial sites for a new, fully automated molecular diagnostics system, the DxN VERIS Molecular Diagnostics System (Beckman Coulter Inc.), including the virology section at the Hospital Clinic of Barcelona, Spain, the department of laboratory medicine at Niguarda Hospital, Milan, Italy, the department of clinical microbiology at the Hospital Universitario 12 de Octubre, Madrid, Spain and the virology department at Sheffield Teaching Hospitals NHS Foundation Trust, UK.
The DxN VERIS System, launched at ECCMID 2015, consolidates nucleic acid extraction, amplification, quantification and detection onto a single automated instrument for a number of molecular targets, including HIV-1, HCV, HBV and CMV. The system offers single sample random access and the potential to improve clinical laboratory workflow efficiency.
The performances of the VERIS assays for CMV, HBV, HCV and HIV-1 were evaluated using standard and control samples, as well as clinical samples, and were compared to various existing viral load methods in each laboratory. In addition, a series of independent time/workflow analysis studies were performed by Nexus Global Solutions (Plano, Texas, USA).
Productivity and workflow improvements
The results of the comparative workflow studies performed in these participating laboratories are summarized below:
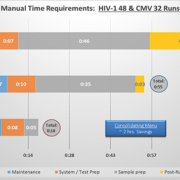
- The DxN VERIS System workflow involved far fewer steps and consumables than existing methods, particularly in the pre-analytical phase, and required reduced hands-on time, which resulted in significant savings in the time that staff are tied to the process (examples shown in Figure 1).
- Significant improvement in time to first result. This is in contrast to the current methods, where results are not available until the end of the assay run.
- The DxN VERIS System allows true, single sample random access which, combined with short assay runtimes, ensures the rapid turnaround of results.
- The DxN VERIS system allowed much faster turnaround of results in a normal working week, with all results being reported within 8-24 hours of receipt (depending on the laboratory), unlike existing methods which often required several days (example in Figure 2).
Duncan Whittaker, Laboratory Manager Virology at Sheffield Teaching Hospitals NHS Foundation Trust, shared his experience:
“Hands on time requirements were measured specifically for the HIV-1 and CMV assays. If these two assays alone were consolidated onto the DxN VERIS it would save around 2 hours manual time per day. If all four parameters were consolidated onto the DxN VERIS system, it is estimated that this would ultimately save at least 0.6 whole time equivalent (WTE) biomedical scientists.”
Diana Fanti, Molecular Biology Laboratory Manager at Niguarda Hospital, Milan, commented:
“By reducing manual intervention and automating processes from sample loading to reporting of results, the DxN VERIS offers the potential to transform clinical laboratory workflows. Each assay is supplied in a unique, single cartridge system, and all consumables and reagents are stored on-board, which cuts preparation time compared to alternative methods”.
Rafael Delgado, Head of Clinical Microbiology at the Hospital Universitario 12 de Octubre, Madrid, agreed:
“One of the most important aspects of the system for our laboratory is the ability to process samples as they are received in the laboratory. With our pervious method, we had to work in batches of 24 or 48, collecting and storing samples throughout the day (or overnight) until we had sufficient for a single run. Then results were not available until the entire run was completed. Now, with the random access capabilities of the DxN VERIS system, this has changed. We receive samples around the clock and we are able to run them straight away. This has improved our response times significantly, from 24-28 hours to just 4-5 hours from sample receipt, and with comparable quality of results compared to our previous method.”
Driving efficiency
DxN VERIS assays are supplied in a unique single cartridge system, which saves further preparation time and effort compared to alternative methods.
The DxN VERIS System also allows more efficient use of staff. Dr Fanti commented:
“By transforming laboratory organization and workflows and reducing manual intervention, viral loads (which account for about 50% of the molecular workload) could be completed in a single day using the DxN VERIS. Requiring fewer people to be dedicated to this purpose, this makes it possible to accomplish more work with the same number of staff.”
Duncan Whittaker agreed, stating:
“The ease of use of the DxN VERIS would help to address staffing issues, as routine operation could be performed by medical laboratory assistants, allowing biomedical scientists to be redeployed more effectively in more skilled areas. Training staff to use the DxN VERIS is very quick and straightforward, taking just 20 minutes. Furthermore, as there is less hands on intervention required, the laboratory could achieve more without any increase in staff. With an annual cost improvement package to meet, anything that helps to increase productivity is a bonus.”
Rafael Delgado also appreciated the benefits for laboratory staff, commenting:
“The DxN VERIS system has been well received by laboratory staff and has expanded our service capabilities. Fully automated from the loading of samples to obtaining results, it is easy to operate by laboratory technicians of all abilities. In addition, since it involves minimal manual intervention and fewer steps than our previous method, there is less opportunity for error and staff have more time to perform other important tasks in the laboratory.”
Conclusions
In conclusion, Duncan Whittaker continued:
“Following the workflow analysis study, it was apparent that the improved workflow and time savings that can be achieved using the DxN VERIS Molecular Diagnostics System could have an enormous impact on the challenges faced by our laboratory. In terms of addressing increasing workloads, the reduced manual intervention required for DxN VERIS would allow more work to be performed per member of staff. The more efficient workflow would free staff to perform other tasks, which would allow the laboratory to develop new services and further increase the department’s test repertoire. This, and improved turnaround times, would help the laboratory to remain competitive in an increasingly competitive environment.”
Since early 2016, the Clinical Microbiology department at the Hospital Universitario 12 de Octubre, Madrid, has also been using the DxN VERIS System routinely for HBV and HCV viral load quantifications. Rafael Delgado commented:
“Our experiences in evaluating the DxN VERIS system enabled us to appreciate its potential as an enabler for an improved molecular biology clinical service. The increased automation and random access offer workflow improvements that simplify laboratory tasks and reduce the potential for human error. Furthermore, its overall performance and ease of use facilitated the smooth introduction of the technology in our laboratory.”
“Our annual volume of HBV and HCV samples is around 7,000 and, as a clinical laboratory working closely alongside medical staff, our viral load results support timely clinical decision making and subsequent patient management. In this respect the DxN VERIS system is ideal for our needs, providing same day results to our outpatient clinics.”
Beckman Couter is commited to providing an increasing menu of assays for the DxN VERIS Molecular Diagnostics System.
Email: info@beckmanmolecular.com or visit www.beckmancoulter.com/moleculardiagnostics
Contributors
Professor Jordi Vila, Head of Department of Clinical Microbiology and Dr Angeles Marcos, Head of the Virology Section
Hospital Clinic, School of Medicine
University of Barcelona, Spain
Providing a full range of medical and surgical specialties for a local population of over half a million, the Hospital Clinic of Barcelona is also a National and International Centre of reference. The Hospital’s Department of Clinical Microbiology, also a reference laboratory for organ transplantation, operates 24 hours a day, seven days a week and, like many laboratories throughout Europe, has experienced increasing workloads in recent years. HBV HCV CMV and HIV-1 viral loads constitute an annual workload volume of nearly 19,000 tests.
Diana Fanti, Molecular Biology Laboratory Manager
Department of Laboratory Medicine, Niguarda Hospital, Milan, Italy
Niguarda Hospital in Milan is one of Italy’s leading General Hospitals, and provides an extensive range of medical disciplines for adults and children throughout the Lombardy region and beyond. The hospital’s Department of Laboratory Medicine aims to offer a complete, continuous and prompt diagnostic laboratory testing service and is committed to research into automation and analysis to ensure this is maintained. Its busy Molecular Biology Laboratory performed an estimated 40,000 tests in 2015, which is approximately 10% increase on the previous year.
Rafael Delgado, Head of Clinical Microbiology
Hospital Universitario 12 de Octubre, Madrid, Spain
With 1,300 beds and over 6,000 employees, the Hospital Universitario 12 de Octubre in Madrid is one of the largest hospitals in Spain, serving a population of more than 500,000 people in and around the capital. It is an important teaching and research center with a number of areas of expertise, including organ transplantation and the diagnosis and treatment of cancer. The hospital’s Clinical Microbiology Department has a significant serology workload, processing more than 250 serology samples every day, which includes viral load testing for targets such as cytomegalovirus (CMV), hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus type 1 (HIV-1).
Duncan Whittaker, Laboratory Manager Virology
Sheffield Teaching Hospitals NHS Foundation Trust
The Department of Virology at Sheffield Teaching Hospitals NHS Foundation Trust provides a valuable diagnostic testing service to the people of Sheffield, serving the local community and five teaching hospitals within the trust as well as the Sheffield Children’s Hospital. It is also a referral laboratory receiving samples from further afield for a variety of tests, including routine viral loads and molecular diagnostics. The department’s annual automated workload includes around 105,000 serology samples (more than 300,000 tests) and 65,000 samples for molecular testing (around 129,000 tests), as well as 60,000 samples for Chlamydia and Gonorrhoea testing.