Improving diagnostic accuracy and laboratory test result interpretation in children and adolescents
Appropriate reference intervals are critical for interpretation of laboratory test results and accurate assessment of health and disease. However, pediatric reference intervals are severely lacking, leading to significant risk of misdiagnosis. CALIPER has addressed these gaps by establishing a robust reference interval database based on thousands of healthy children and adolescents.
by Victoria Higgins and Dr Khosrow Adeli
Introduction
The clinical laboratory provides objective data through laboratory testing of bodily fluids (e.g. serum, plasma) to aid in several aspects of medical decision making, including identifying risk factors and symptoms, diagnosing disease, and monitoring treatment. To correctly interpret laboratory test results, they are often compared to reference intervals (RIs), sometimes referred to as ‘normative’ or ‘expected’ values. RIs are commonly defined as the central 95% of the distribution of laboratory test results expected in a healthy, reference population [1]. Laboratory values that fall outside the appropriate RI may be interpreted as abnormal, possibly indicating the need for additional medical follow-up and/or treatment [2]. Given their critical importance to healthcare it would be expected that accurate RIs, appropriate for the patient population, are used in clinical practice. However, this is unfortunately far from the truth.
Importance of pediatric reference intervals
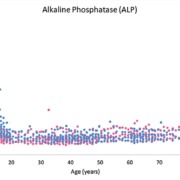
It can be challenging and costly for individual laboratories to develop RIs for their specific patient population, due to the necessity of recruiting a sufficiently large number of healthy individuals [i.e. The Clinical Laboratory Standards Institute (CLSI) recommends 120 individuals per partition] [1]. This is particularly true for pediatrics, a population in which unique RIs are of high importance. To interpret pediatric test results, laboratories often use RIs that were established on an adult reference population. The use of adult RIs to interpret pediatric test results can lead to erroneous and inaccurate interpretation. This is highlighted in Figure 1, which depicts the concentration of alkaline phosphatase (ALP) throughout pediatric, adult and geriatric age. It is evident that the pediatric population has vastly unique normative ALP values. Unique analyte concentrations in pediatrics is also true for sex hormones, growth hormones and several other analytes [3–5]. Therefore, children should not be viewed as small adults in the context of medical practice, but require separate RIs (i.e. partitions) for different age and/or sex groups, in addition to neonates and premature babies [5].
Closing the gaps in pediatric reference intervals
The current CLSI guidelines, which are mostly focused on adult RIs, acknowledge the special challenges of establishing age- and sex-specific pediatric RIs and recommend development of new initiatives to address the current gaps. The quality of a RI critically depends on the selected reference population. Therefore, the direct method of establishing RIs, which involves recruiting healthy individuals and applying exclusion criteria to select an appropriate reference population, is recommended over the indirect method, which involves using an already existing database (e.g. laboratory information system) to calculate RIs [1]. It is imperative for RI initiatives to focus on recruiting a sufficiently large and healthy reference population to accurately establish appropriate RIs for the pediatric population (i.e. using the direct method). Recognizing the critical need to establish pediatric RIs, several national initiatives have collected health information and blood samples from healthy pediatric populations. These initiatives include KiGGS in Germany [6], the Lifestyle of Our Kids (LOOK) study in Australia [7], CHILDx in the United States [8–10], The COPENHAGEN Puberty Study in the Nordic countries [11], and The Canadian Laboratory Initiative on Pediatric RIs (CALIPER) in Canada [5, 12].
The KiGGS initiative collected comprehensive, nationwide data on the health status of over 17 000 children and adolescents aged 0 to 17 years, across 167 locations in Germany [6]. This study has focused on laboratory parameters of general health indices, markers of nutritional status, immunization status, iron metabolism, thyroid, and indices of atopic sensitization. They have published age-dependent percentiles (3rd to 97th) in German, which may serve as a basis for RIs [13]. The LOOK study in Australia developed age-specific RIs for 37 chemistries, immunoassays, and derived parameters [7]. The CHILDx study was initiated in 2002 at ARUP (Associated Regional and University Pathologists) Laboratories and established RIs for 35 markers for children aged 6 months to 6 years and 58 markers for children aged 7–17 years [8–10]. The Nordic countries have also successfully established pediatric RIs for 21 biochemical properties using samples from healthy children and adolescents aged 5–19 years collected from schools from 2006–2008 in the Copenhagen area in Denmark as part of The COPENHAGEN Puberty Study [11]. However, arguably the most successful initiative has been the CALIPER project in Canada.
CALIPER project
The CALIPER project was initiated by The Paediatric Focus Group of the Canadian Society of Clinical Chemists (CSCC) and primarily based at The Hospital for Sick Children in Toronto (ON, Canada). CALIPER has recruited over 9 000 healthy children and adolescents from schools and community centres to participate at blood collection clinics by completing a health questionnaire, body measurements and donating a blood sample. Using this biobank of healthy pediatric samples, CALIPER has established age-, sex- and, for some biomarkers, Tanner Stage-specific pediatric RIs for over 100 biomarkers including, common biochemical markers, protein markers, lipids and enzymes [12], specialty endocrine markers [14], fertility hormones [15], cancer biomarkers [16], vitamins [17], metabolic disease biomarkers [18], testosterone indices [19] and specialized biochemical markers [20, 21]. All RIs were established in accordance with CLSI guidelines, including sample size requirements, outlier removal, statistical method for partitioning, as well as RI and confidence interval calculations [1].
The majority of RIs were established using Abbott ARCHITECT assays, initially limiting the direct applicability of the CALIPER database to all Canadian laboratories. CALIPER subsequently performed a series of transference and verification studies to expand the CALIPER database to additional assays commonly used in clinical laboratories, including Beckman, Ortho, Roche and Siemens [22–25]. Again, CALIPER performed these studies in accordance with CLSI guidelines and, in fact, often exceeded the sample size and statistical criteria requirements [1, 26]. The comprehensive CALIPER pediatric RI database is available online (www.caliperproject.ca), as well as through a mobile application (CALIPERApp) available on iTunes and Google Play. These tools allow the CALIPER database to be easily accessible to laboratory professionals, physicians, parents and patients.
Continued improvement in pediatric laboratory test interpretation
While significant improvements have been made in pediatric laboratory test interpretation over the past decade, several gaps remain. First, RI data for neonates (including premature babies) and infants (age 0 to <1 year) remains a challenge, owing to difficulties accessing a healthy neonate and infant population. However, the limited neonatal and infantile reference data CALIPER has collected highlights the profound differences in the newborn period, necessitating accurate RIs for this age group. For example, Figure 2 shows the dynamic concentration of creatinine throughout the pediatric age range, particularly the elevated and highly variable levels in the first two weeks of life. A large-scale, comprehensive study aimed at recruiting healthy neonates and infants is required to fill this gap. CALIPER is currently initiating a study with the aim of establishing a complete RI database for neonates and infants, which will greatly improve neonatal healthcare for premature babies, newborns, and infants from primary to complex, tertiary care pediatric centres.
Secondly, the effect of ethnicity on biomarker concentration remains to be comprehensively examined. The International Federation of Clinical Chemistry (IFCC) recommends that every country establishes RIs [27]; however, most nations adopt RIs from studies predominately performed in Western countries based on primarily Caucasian populations without considerations of ethnic differences. Although the majority of biomarkers do not differ between individuals of different ethnic backgrounds, a preliminary examination of the influence of ethnicity in pediatrics by CALIPER has shown that some biomarkers do significantly differ among ethnic groups, including immunoglobulin G (IgG), transferrin, ferritin, and follicle-stimulating hormone (FSH) [12, 14, 15]. Another study examined the influence of ethnicity in adults and found that serum creatine kinase (CK) activity is significantly higher for those of African ancestry. As elevated CK activity is an indicator of statin-induced myopathy, elevated CK activity in those of African ancestry could result in inappropriate discontinuation of statin therapy if ethnic-specific RIs are not used [28]. Another recent study used data from the National Health and Nutrition Examination Survey (NHANES) to develop racial/ethnic-specific RIs among Asians, Blacks, Hispanics, and Whites [29]. CALIPER has initiated a new study to robustly determine the effect of ethnicity on the concentration of routine serum biomarkers by examining and comparing reference values in the four major Canadian ethnic groups (i.e. Caucasian, South Asian, East Asian, and Black).
Lastly, as clinical laboratories adopt their RIs from numerous different sources, including textbooks, manufacturer product inserts, expert opinions, or published literature, RIs in clinical practice may vary substantially between laboratories. A national survey performed in Australia by the Australian Association of Clinical Biochemists (AACB) Harmonisation Group highlights the extensive variation in adult RIs used in clinical practice, which greatly compromises the consistency and reliability of laboratory test result interpretation and patient care [30]. A recent Canadian RI study (manuscript submitted; Adeli K, et al. 2017) by the CSCC Harmonized RI (hRI) Working Group, further highlights the considerable variation in RIs across laboratories with a greater variation observed in pediatric RIs in current clinical use, even between clinical laboratories using the same instrument. These surveys highlight the critical need for harmonized RIs in clinical practice. Initiatives in the Nordic countries [31], UK [32], Australia [33] and Japan [34] have already established harmonized RIs for a number of laboratory tests primarily for adults, but also for pediatrics. The CSCC hRI Working Group is now also working towards Canada-wide RI harmonization.
Conclusion
Children cannot be viewed as small adults and indeed require pediatric-specific RIs appropriately partitioned by age and sex for accurate laboratory test result interpretation. Several national initiatives have begun to address these critical gaps over the past decade by establishing age-, sex- and Tanner Stage-specific RIs for several major analytical platforms. The CALIPER initiative in Canada has arguably been the most comprehensive study to date, with clinical laboratories in several countries globally implementing the CALIPER database into clinical practice. Despite the significant strides recently achieved, further research is warranted in several areas including the establishment of RIs specific to the neonatal and infantile period, ethnic-specific RI for a subset of laboratory markers, and RI harmonization. Collectively, the comprehensive reference database published by CALIPER and the emerging data from ongoing studies directly address the evidence gap in pediatric RIs and contribute to evidence-based interpretation of laboratory test results and enhanced diagnostic accuracy of laboratory biomarkers in current clinical practice.
References
1. Defining, establishing, and verifying RIs in the clinical laboratory; approved guidelines – third edition CLSI document C28-A3. Clinical and Laboratory Standards Institute (CLSI); 2008.
2. Jung B, Adeli K. Clinical laboratory RIs in pediatrics: the CALIPER initiative. Clin Biochem 2009; 42(16–17): 1589–1595.
3. Adeli K, Higgins V, Nieuwesteeg M, Raizman JE, Chen Y, Wong SL, Blais D. Biochemical marker reference values across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult RIs on the basis of the Canadian Health Measures Survey. Clin Chem 2015; 61(8): 1049–1062.
4. Adeli K, Higgins V, Nieuwesteeg M, Raizman JE, Chen Y, Wong SL, Blais D. Complex reference values for endocrine and special chemistry biomarkers across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult RIs on the basis of the Canadian Health Measures Survey. Clin Chem 2015; 61(8): 1063–1074.
5. Shaw JLV, Binesh Marvasti T, Colantonio D, Adeli K. Pediatric RIs: challenges and recent initiatives. Crit Rev Clin Lab Sci 2013; 50(2): 37–50.
6. Kohse KP. KiGGS – the German survey on children’s health as data base for RIs and beyond. Clin Biochem 2014; 47(9): 742–743.
7. Southcott EK, Kerrigan JL, Potter JM, Telford RD, Waring P, Reynolds GJ, Lafferty ARA, Hickman PE. Establishment of pediatric RIs on a large cohort of healthy children. Clin Chim Acta 2010; 411(19–20): 1421–1427.
8. Flanders MM, Crist RA, Roberts WL, Rodgers GM. Pediatric RIs for seven common coagulation assays. Clin Chem 2005; 51(9): 1738–1742.
9. Clifford SM, Bunker AM, Jacobsen JR, Roberts WL. Age and gender specific pediatric RIs for aldolase, amylase, ceruloplasmin, creatine kinase, pancreatic amylase, prealbumin, and uric acid. Clin Chim Acta 2011; 412(9–10): 788–790.
10. Johnson-Davis KL, Moore SJ, Owen WE, Cutler JM, Frank EL. A rapid HPLC method used to establish pediatric RIs for vitamins A and E. Clin Chim Acta 2009; 405(1–2): 35–38.
11. Hilsted L, Rustad P, Aksglæde L, Sørensen K, Juul A. Recommended Nordic paediatric RIs for 21 common biochemical properties. Scand J Clin Lab Invest 2013; 73(1): 1–9.
12. Colantonio DA, Kyriakopoulou L, Chan MK, Daly CH, Brinc D, Venner AA, Pasic MD, Armbruster D, Adeli K. Closing the gaps in pediatric laboratory RIs: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem 2012; 58(5): 854–868.
13. Dortschy R, Schaffarth Rosario A, Scheidt-Nave C, Thierfelder W, Thamm M, Gutsche J. Bevölkerungsbezogene Verteilungswerte ausgewählter Laborparameter aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS). Beiträge zur Gesundheitsberichterstattung des Bundes. Berlin: Robert Koch-Institut; 2009.
14. Bailey D, Colantonio D, Kyriakopoulou L, Cohen AH, Chan MK, Armbruster D, Adeli K. Marked biological variance in endocrine and biochemical markers in childhood: establishment of pediatric RIs using healthy community children from the CALIPER cohort. Clin Chem 2013; 59(9): 1393–1405.
15. Konforte D, Shea JL, Kyriakopoulou L, Colantonio D, Cohen AH, Shaw J, Bailey D, Chan MK, Armbruster D, Adeli K. Complex biological pattern of fertility hormones in children and adolescents: a study of healthy children from the CALIPER cohort and establishment of pediatric RIs. Clin Chem 2013; 59(8): 1215–1227.
16. Bevilacqua V, Chan MK, Chen Y, Armbruster D, Schodin B, Adeli K. Pediatric population reference value distributions for cancer biomarkers and covariate-stratified RIs in the CALIPER cohort. Clin Chem 2014; 60(12): 1532–1542.
17. Raizman JE, Cohen AH, Teodoro-Morrison T, Wan B, Khun-Chen M, Wilkenson C, Bevilaqua V, Adeli K. Pediatric reference value distributions for vitamins A and E in the CALIPER cohort and establishment of age-stratified RIs. Clin Biochem 2014; 47(9): 812–815.
18. Teodoro-Morrison T, Kyriakopoulou L, Chen YK, Raizman JE, Bevilacqua V, Chan MK, Wan B, Yazdanpanah M, Schulze A, Adeli K. Dynamic biological changes in metabolic disease biomarkers in childhood and adolescence: a CALIPER study of healthy community children. Clin Biochem 2015; 48(13–14): 828–836.
19. Raizman JE, Quinn F, Armbruster DA, Adeli K. Pediatric RIs for calculated free testosterone, bioavailable testosterone and free androgen index in the CALIPER cohort. Clin Chem Lab Med 2015; 53(10): e239–243.
20. Kelly J, Raizman JE, Bevilacqua V, Chan MK, Chen Y, Quinn F, Shodin B, Armbruster D, Adeli K. Complex reference value distributions and partitioned RIs across the pediatric age range for 14 specialized biochemical markers in the CALIPER cohort of healthy community children and adolescents. Clin Chim Acta 2015; 450: 196–202.
21. Karbasy K, Lin DCC, Stoianov A, Chan MK, Bevilacqua V, Chen Y, Adeli K. Pediatric reference value distributions and covariate-stratified RIs for 29 endocrine and special chemistry biomarkers on the Beckman Coulter Immunoassay Systems: a CALIPER study of healthy community children. Clin Chem Lab Med 2016; 54(4): 643–657.
22. Estey MP, Cohen AH, Colantonio DA, Chan MK, Marvasti TB, Randell E, Delvin E, Cousineau J, Grey V, et al. CLSI-based transference of the CALIPER database of pediatric RIs from Abbott to Beckman, Ortho, Roche and Siemens Clinical Chemistry Assays: direct validation using reference samples from the CALIPER cohort. Clin Biochem 2013; 46(13–14): 1197–1219.
23. Higgins V, Chan MK, Nieuwesteeg M, Hoffman BR, Bromberg IL, Gornall D, Randell E, Adeli K. Transference of CALIPER pediatric RIs to biochemical assays on the Roche cobas 6000 and the Roche Modular P. Clin Biochem 2016; 49(1–2): 139–149.
24. Araújo PAT, Thomas D, Sadeghieh T, Bevilacqua V, Chan MK, Chen Y, Randell E, Adeli K. CLSI-based transference of the CALIPER database of pediatric RIs to Beckman Coulter DxC biochemical assays. Clin Biochem 2015; 48(13–14): 870–880.
25. Abou El Hassan M, Stoianov A, Araújo PAT, Sadeghieh T, Chan MK, Chen Y, Randell E, Nieuwesteeg M, Adeli K. CLSI-based transference of CALIPER pediatric RIs to Beckman Coulter AU biochemical assays. Clin Biochem 2015; 48(16–17): 1151–1159.
26. Method comparison and bias estimation using patient samples; approved guidelines – second edition CLSI document EP9-A2. Clinical and Laboratory Standards Institute (CLSI) 2002.
27. Solberg HE, Stamm D. IFCC recommendation: The theory of reference values. Part 4. Control of analytical variation in the production, transfer and application of reference values. J Autom Chem 1991; 13(5): 231–234.
28. Brewster LM, Mairuhu G, Sturk A, van Montfrans GA. Distribution of creatine kinase in the general population: implications for statin therapy. Am Heart J 2007; 154(4): 655–661.
29. Lim E, Miyamura J, Chen JJ. Racial/ethnic-specific RIs for common laboratory tests: a comparison among Asians, Blacks, Hispanics, and White. Hawaii J Med Public Health 2015; 74(9): 302–310.
30. Jones GR, Koetsier SD. RCPAQAP first combined measurement and RI survey. Clin Biochem Rev 2014; 35(4): 243–250.
31. Rustad P, Felding P, Franzson L, Kairisto V, Lahti A, Mårtensson A, et al. The Nordic Reference Interval Project 2000: recommended RIs for 25 common biochemical properties. Scand J Clin Lab Invest 2004; 64(4): 271–284.
32. Berg J, Lane V. Pathology harmony; a pragmatic and scientific approach to unfounded variation in the clinical laboratory. Ann Clin Biochem 2011; 48(3): 195–197.
33. Tate JR, Sikaris KA, Jones GR, Yen T, Koerbin G, Ryan J, Reed M, Gill J, Koumantakis G, et al. Harmonising adult and paediatric RIs in Australia and New Zealand: an evidence-based approach for establishing a first panel of chemistry analytes. Clin Biochem Rev 2014; 35(4): 213–235.
34. Yamamoto Y, Hosogaya S, Osawa S, Ichihara K, Onuma T, Saito A, Banba K, Araki H, Nagamine Y, et al. Nationwide multicenter study aimed at the establishment of common RIs for standardized clinical laboratory tests in Japan. Clin Chem Lab Med 2013; 51(8): 1663–1672.
The authors
Victoria Higgins PhD candidate; Khosrow Adeli* PhD, FCACB, DABCC, FACB
CALIPER program, Pediatric Laboratory Medicine, The Hospital for Sick Children, University of Toronto, Toronto, ON M5G 1X8, Canada
*Corresponding author
E-mail: khosrow.adeli@sickkids.ca