LC-MS/MS in clinical diagnostic laboratories: screening for catecholamine-producing tumours
Accurate quantitative targeted analysis of low molecular weight compounds is one of the most important needs in clinical diagnostic laboratories. The enhanced analytical specificity and sensitivity of modern liquid chromatography–tandem mass spectrometry based methods satisfy this requirement for screening of endocrine related disorders, including those affecting steroidogenic systems or overproduction of catecholamines.
by Dr M. Peitzsch and Professor G. Eisenhofer
Liquid chromatography – tandem mass spectrometry (LC-MS/MS)
The development of electrospray ionization (ESI) enabled the introduction of aqueous chromatographic eluates into mass spectrometers, an advance for which John Bennett Fenn was awarded the Nobel Prize in chemistry in 2002. Subsequent refinements in liquid chromatography coupled with ESI mass spectrometry led to analytical applications directed at a broad range of macromolecules from peptides, proteins, glycoproteins and glycolipids to lower molecular weight polar and non-polar compounds, including fatty acids, vitamins, nucleic acids, steroids, amino acids and biogenic amines.
Introduction of tandem mass spectrometry (MS/MS) represented a further breakthrough enabling analyses of relationships between ‘parent or precursor ions’ in the first stage and ‘daughter or product ions’ in the second stage of the instrument [1]. For targeted quantitative analyses, the filtering capabilities and the multiple reaction monitoring (MRM) possible through MS/MS triple quadrupole instruments provide not only high selectivity, but also improved signal-to-noise ratios. In recent years, the increasing commercial availability of stable isotope labelled substances, used as internal standards, has facilitated the application of stable isotope dilution internal standardization as the gold standard for accurate quantitative analyses. Since the physicochemical properties of the target analyte and the stable-isotope-labelled internal standard are similar, this approach compensates for all variations which occur during sample extraction, injection, chromatography, ionization, and ion detection with dow stream improvements in analytical precision and accuracy [2].
The high analytical specificity of LC-MS/MS allows less rigorous sample purification and chromatographic resolution than for standard high performance liquid chromatographic (HPLC) procedures employing ultraviolet, electrochemical or fluorimetric detection. This, and other developments in column chemistry, such as those allowing ultra-high performance liquid chromatography (UPLC), in turn enables higher sample throughput than offered by conventional HPLC procedures. Fusion of LC-MS/MS with other technologies, such as multiplexing parallel LC systems and turbo-flow technology, provides additional advantages for efficient and accurate high-throughput quantitative analyses. Further, automated online sample extraction systems minimize time spent on sample preparation and allow multiple applications to be efficiently handled by one instrument.
All the above possibilities for extending sample throughput, combined with the versatility of a single LC-MS/MS system to take over the jobs of multiple standard HPLC systems, provide advantages that justify the initial high cost of the instrument. Recognized impediments to implementing LC-MS/MS technology include the complexity of the instrumentation associated with the necessity for highly skilled personnel, especially for method development. A lack of standardization combined with a shortage of inter-laboratory comparison programs for quality assurance represent other limitations to acceptance by clinical laboratories.
LC-MS/MS in clinical diagnostics laboratories
The improvements in precision and accuracy offered by LC-MS/MS are now well recognized as offering critical advances over standard HPLC and immunoassay procedures, which are subject to analytical interferences or do not allow precise and accurate identification of structurally-related compounds, such as steroid hormones. Such advances are important to the fields of endocrinology and clinical laboratory medicine where accurate quantitative analysis is crucial for diagnostic purposes [2].
LC-MS/MS applications are now used in clinical and forensic toxicology, such as for drugs-of-abuse testing. In clinical laboratory medicine, LC-MS/MS is used for measurements of endocrine hormones such as steroids, biogenic amines and thyroid hormones, as well as for therapeutic drug monitoring and in new-born screening for assessment of inborn errors of metabolism.
In contrast to commonly used immunoassays, LC-MS/MS enables measurements of multiple analytes for each sample processed. Such determination of analyte profiles includes those for the various thyroid hormones, different vitamin D metabolites and steroid profiles, all available in single analytical runs. Profiles of steroid hormones, although mainly used in research applications, hold considerable promise for the routine clinical assessment of a wide range of
steroidogenic disorders.
LC-MS/MS based screening for catecholamine producing tumours
Pheochromocytomas and paragangliomas (PPGLs) are tumours arising respectively in adrenal and extra-adrenal chromaffin cells that are characterized by an overproduction of catecholamines. Without diagnosis and an appropriate treatment, the excessive secretion of catecholamines by PPGLs can lead to disastrous consequences.
For initial biochemical screening different tests are available, including plasma or urinary measurements of the catecholamines – norepinephrine, epinephrine and dopamine – and their respective O-methylated metabolites – normetanephrine, metanephrine and 3-methoxytyramine. Whereas the free metabolites are usually measured in plasma, analyses in urine are commonly performed after acid hydrolysis in which free metabolites are liberated from sulfate conjugates.
In 2002, Taylor and Singh presented an LC-MS/MS method for the analysis of deconjugated urinary fractionated metanephrines [3]. The outlined advantages of this method over other methods, such as immunoassay and HPLC-ECD (electrochemical detection), included relative freedom from drug interferences, high sample throughput and short chromatographic run times. Subsequently, there has been a plethora of related methods published, including many that enable detection of the much lower concentrations of plasma free metanephrines than urinary deconjugated metanephrines.
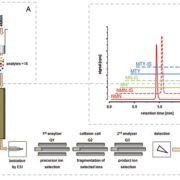
Development of new sample preparation procedures, either offline or online to the LC-MS/MS system, have been particularly useful for automated high-throughput procedures [4, 5]. More recent improvements in LC-MS/MS instrumentation have led to improved analytical sensitivity, now even enabling accurate and precise measurements of picomolar plasma concentrations of 3-methoxytyramine, the O-methylated metabolite of dopamine [5–7]. This valuable biomarker not only allows detection of dopamine producing PPGLs, but can also be used to detect malignancy [9]. Using LC-MS/MS, the diagnostic performance of 3-methoxytyramine as a marker of malignancy was characterized by an enhanced diagnostic sensitivity of 86% and specificity of 96% [8] [Fig. 1].
Problems with drug interferences in HPLC-ECD and immunoassay-based methods are largely overcome using LC-MS/MS. For example, problems of acetaminophen (paracetamol) interferences in HPLC-ECD procedures are not a problem for LC-MS/MS [8, 10]. Chromatographic disruptions associated with certain disorders, such as renal insufficiency, are also less of a problem by LC-MS/MS than by HPLC-ECD [8].
Use of plasma free normetanephrine, metanephrine and methoxytyramine for reliable diagnosis of PPGLs requires collection of blood samples after 30 minutes of supine rest and an overnight fast. These conditions pose difficulties for many clinicians, which can result in excessive false-positive results or worse, missed diagnoses when inappropriately high upper cut-offs have been derived from seated sampling. Measurements of urinary metanephrines provide a reasonable alternative test for those situations where blood samples cannot be collected appropriately.
As mentioned above, urinary metanephrines are commonly measured after an acid-hydrolysis deconjugation step. This procedure is based mainly on historical convention, where initially less sensitive instruments did not allow measurements of the much lower urinary concentrations of free rather than deconjugated metanephrines. Improvements in analytical sensitivity now, however, allow analysis of urinary free metanephrines, [11, 12]. Unlike the sulfate-conjugated derivatives, which are produced by a sulfotransferase enzyme located in the gastrointestinal tract, the free metabolites are produced within chromaffin cells. This provides a potential advantage for measurements of the free metabolites. Another advantage is that there are no suitable quality controls or calibrators for measurements of urinary deconjugated metanephrines [12, 13]. Those that are available are almost entirely in the free form so that procedures will always pass quality control even if the deconjugation step is missed and values for patient samples are grossly under-estimated. Measurements of urine free metanephrines avoid this potential pitfall in quality assurance.
Finally, with measurements of urinary free metanephrines it is possible to combine the measurements with urinary catecholamines in a single run [12;14]. This also provides an advantage over measurements of urinary deconjugated metanephrines, where the deconjugation step does not allow measurements of free catecholamines.
The difficulties in applying LC-MS/MS in the clinical chemistry laboratory, such as associated with high initial instrument costs and need for expertise, are easily overshadowed by the analytical advantages. High sample throughput and the analytical versatility offered by LC-MS/MS, which enables rapid method switching, in particular represent important advantages over standard HPLC methods. Nevertheless, such advantages are not easily realized by the small hospital-based laboratory where high sample throughput is not an important consideration. In the US the highly competitive nature of the heath care system is an incentive for centralized testing where efficiency and low operating costs associated with high sample throughput (economy of scale) are more easily realized. In the US the switch from immunoassays or HPLC-based methodology to superior LC-MS/MS technology is therefore likely to remain more advanced than in Europe.
Summary and conclusion
Modern LC-MS/MS systems provide well-recognized accuracy for quantitative targeted measurements of analytes used for clinical diagnostics. The high-throughput capabilities and versatility of LC-MS/MS instrumentation enable multiple applications for rare diseases to be handled by a single instrument. Furthermore, single analyte assays can be extended to accurate profiling by LC-MS/MS assays, providing deeper insight into endocrine metabolic disorders. This, however, remains largely a research-based application and for LC-MS/MS to be readily adapted for routine use in the clinical laboratories, other advantages such as those associated with economy of scale must be appreciated and realized.
References
1. Glish GL, Vachet RW. The basics of mass spectrometry in the twenty-first century. Nature Reviews Drug Discovery 2003; 2: 140–150.
2. Vogeser M, Parhofer KG. Liquid chromatography tandem-mass spectrometry (LC-MS/MS) – Technique and applications in endocrinology. Exp Clin Endocrinol Diabetes 2007; 115: 559–570.
3. Taylor RL, Singh RJ. Validation of liquid chromatography-tandem mass spectrometry method for analysis of urinary conjugated metanephrine and normetanephrine for screening of pheochromocytoma. Clinical Chemistry 2002; 48: 533–539.
4. Lagerstedt SA, O’Kane DJ, et al. Measurement of plasma free metanephrine and normetanephrine by liquid chromatographym-tandem mass spectrometry for diagnosis of pheochromocytoma. Clinical Chemistry 2004; 50: 603–611.
5. Peaston RT, Graham KS, et al. Performance of plasma free metanephrines measured by liquid chromatography-tandem mass spectrometry in the diagnosis of pheochromocytoma. Clinica Chimica Acta 2010; 411: 546–552.
6. Eisenhofer G, Goldstein DS, et al. Biochemical and clinical manifestations of dopamine-producing paragangliomas: Utility of plasma methoxytyramine. J Clin Endocrinol Metab 2005; 90: 2068–2075.
7. Eisenhofer G, Lenders JW, et al. Plasma methoxytyramine: A n ovel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. European Journal of Cancer 2012; 48(11): 1739–1749.
8. Peitzsch M, Prejbisz A, et al. Analysis of plasma 3-methoxytyramine, normetanephrine and metanephrine by ultra performance liquid chromatography – tandem mass spectrometry: utility for diagnosis of dopamine-producing metastatic phaeochromocytoma. Ann Clin Biochem 2013; 50: 147–155.
9. Eisenhofer G, Tischler AS, et al. Diagnostic Tests and Biomarkers for Pheochromocytoma and Extra-adrenal Paraganglioma: From Routine Laboratory Methods to Disease Stratification. Endocrine Pathology 2012; 23: 4–14.
10. Petteys JB, Graham KS, et al. Performance characteristics of an LC–MS/MS method for the determination of plasma metanephrines. Clinica Chimica Acta 2012; 413: 1459–1465.
11. Boyle JG, Davidson DF, et al. Comparison of diagnostic accuracy of urinary free metanephrines, vanillyl mandelic acid, and catecholamines and plasma catecholamines for diagnosis of pheochromocytoma. J Clin Endocrinol Metab 2007; 92: 4602–4608.
12. Peitzsch M, Pelzel D, et al. Simultaneous liquid chromatography tandem mass spectrometric determination of urinary free metanephrines and catecholamines, with comparisons of free and deconjugated metabolites. Clinica Chimica Acta 2013; 418: 50–58.
13. Simonin J, Gerber-Lemaire S, et al. Synthetic calibrators for the analysis of total metanephrines in urine: Revisiting the conditions of hydrolysis. Clinica Chimica Acta 2012; 413: 998–1003.
14. Whiting MJ. Simultaneous measurement of urinary metanephrines and catecholamines by liquid chromatography with tandem mass spectrometric detection. Ann Clin Biochem 2009; 46: 129–136.
The authors
Mirko Peitzsch*1 PhD and
Graeme Eisenhofer1,2 PhD
1 Institute for Clinical Chemistry and Laboratory Medicine, University Hospital Carl Gustav Carus at the Technical University Dresden, Dresden, Germany
2 Department of Medicine III, University of Dresden, Dresden, Germany
*Corresponding author
E-mail: Mirko.Peitzsch@uniklinikum-dresden.de