Monitoring kidney function in aminoglycoside therapy
Aminoglycosides are antibiotics largely used at the hospital. Their nephrotoxicity imposes therapeutic drug monitoring as well as kidney function monitoring. Creatinine is the most widely used biochemical marker; however, new biomarkers such as neutrophil gelatinase associated lipocalin (NGAL), cystatin C (Cys C) or kidney injury molecule-1 (KIM-1) can allow the detection of acute kidney injury more quickly.
by F. Fraissinet, E. Sacchetto and Dr E. Bigot-Corbel
Background
Aminoglycosides are bactericidal antibiotics used for the treatment of Gram-negative or endocarditis infections. The most important adverse effects of aminoglycosides are nephrotoxicity and ototoxicity. The prevalence of aminoglycoside-associated nephrotoxicity is estimated at 10 to 20 %, although it also depends on the patient’s clinical condition and exposure to nephrotoxic drugs such as cyclosporine, anti-inflammatory or iodinated drugs. In intensive care units, nephrotoxicity is most frequent (60% of patients) and associated with a high rate of mortality [1, 2]. Nephrotoxicity mainly results in nonoliguric acute kidney injury which occurs following 7 to 10 days. This toxicity may manifest as a decrease in the glomerular filtration rate with glycosuria, hypokalemia and hypocalcemia. Aminoglycosides are often administered by intravenous drip and are freely eliminated by glomerular filtration and reabsorbed by the proximal tubule. Of the injected dose, 5% is retained by epithelial cells of the proximal tubule. After endocytosis in tubular cells, these molecules accumulate in lysosomes and induce phospholipidosis, alteration of key cellular components and apoptosis. Aminoglycosides also have glomerular effects: gentamicin stimulates mesangial proliferation, produces mesangial contraction and induces neutralization of negative charges of the glomerulus [3]. Gentamicin also induces a reduction in renal blood flow with an increased renal vascular resistance. These factors contribute to decrease the glomerular filtration rate (GFR). Additional risk factors for nephrotoxicity induced by aminoglycoside have been identified as sepsis, prolonged therapy, renal or liver dysfunction, hypokalemia or hypomagnesemia. Nephrotoxicity is less frequent when aminoglycosides are administered once daily compared with 12 h [4].
Methods of detection of acute kidney injury induced by aminoglycoside therapy
Classic markers: creatinine and creatinine clearance
Creatinine is the most widely used marker in the diagnosis of the acute renal insufficiency. For defining AKI, the Risk Injury Failure Loss End stage kidney disease (RIFLE) classification is based on increase of serum creatinine concentration and decrease of glomerular filtration rate. The introduction of the RIFLE classification has increased the conceptual understanding of AKI syndrome, and this classification has been successfully tested in a number of clinical studies [5].
In spite of an easily accessible dosage, creatinine as a marker of AKI has some drawbacks. Creatinine is filtered by the glomerulus and is not bound to plasma proteins. In standard physiological conditions, the daily rate of creatinine production is constant; however, the rate of creatinine production is affected by conditions of muscular pathology or muscular loss (as occurs in intensive care and cirrhosis). In these patients, AKI does not result in an increase of serum creatinine levels. Other factors, such as age, sex, ethnic group and diet, also influence serum concentrations of creatinine. Creatinine is not the ideal marker to estimate GFR, because it is secreted by renal tubule, which artefacutally increases glomerular filtration rate. At low serum creating concentrations, creatinine is lacks sensitivity to estimate GFR. Large changes in GFR may be associated with relatively small changes in serum creatinine (See Figure 2 in Delanaye et al. [6]). The rise of the creatinine is late (occurring after 3–5 days) and is not specific for nephrotoxicity induced by aminoglycosides, and an increase of creatinine in AKI is a function of the initial concentration of creatinine [7].
To estimate GFR, formulas that use creatinine plasma concentration, such as the Modified Diet in Renal Disease formula (MDRD), Chronic Kidney Disease Epidemiology collaboration formula (CKD-EPI) or estimation of clearance creatinine by Cockroft–Gault (CG) equation, were derived in subjects with chronic, not acute, kidney disease. A limitation of the MDRD equation was an underestimation of GFR in the high range. The CKD-EPI equation performs better at high GFR levels (GFR >60 mL/min/1.73 m²). Use of serum creatinine concentration to estimate GFR supposes a steady-state between creatinine production and excretion [8]. In spite of the use of correction factors, it is more difficult to estimate GFR in Asian or African populations as well as in elderly or obese patients [9].
New biomarkers
Cystatin C
Cystatin C (CysC), a 13-kDa endogenous cysteine proteinase inhibitor, plays an important role in intracellular catabolism of various peptides and proteins. CysC is considered to be a good biomarker of decreased kidney function because it is produced at a relatively constant rate and released into plasma, and is filtered by glomeruli without tubular secretion. The influence of muscular mass is less than for creatinine, and CysC allows diagnosis of AKI 48 h before serum creatinine [10]. Equations with serum CysC concentration can also estimate GFR. If GFR is great than 60 mL/min/m², CysC measurement is more powerful than the MDRD equation. CysC is a useful biomarker for early detection of AKI in the pediatric population and for patients in the intensive care unit, as CysC determination can be performed in serum and/or in urine. In spite of efforts to standardize the procedure, there is no reference method. Production of CysC also depends on hormonal factors, so CysC cannot be used in cases of thyroid dysfunction.
Neutrophil gelatinase associated lipocalin
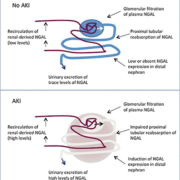
Neutrophil gelatinase associated lipocalin (NGAL) is a protein of 25 kDa protein of the lipocalin family and is covalently bound to matrix metalloproteinase-9. NGAL is expressed early in ischemic kidney impairment in animal models. During AKI, NGAL expression is induced in distal nephron epithelia resulting in elevated plasma and urinary levels of NGAL (Fig. 1) [2]. NGAL determination can be performed on serum and/or urine by immunoturbimetric or immunofluorimetric assays. There is a general agreement on a cut-off value of >150 ng/mL, but a clear cut-off NGAL concentration for AKI has not been reported. Several studies show the importance of NGAL in cardiac surgery or critically ill patients for predicting AKI. NGAL is also useful for the detection of nephrotoxicity induced by contrast agents and has prognostic value for mortality or initiation of renal replacement therapy. Plasma NGAL measurements may be influenced by a number of coexisting variables as chronic hypertension, systemic infections, inflammatory conditions or hypoxia. Changes in NGAL values are potentially associated with septic state or aminoglycoside therapy [11].
Kidney injury molecule-1
Kidney injury molecule-1 (KIM-1) is a glycoprotein localized in the apical membrane of the proximal tubule of kidney, and KIM-1 expression can be induced by nephrotoxic drugs. Urine KIM-1 is a promising biomarker of proximal tubular injury. As with NGAL, urinary KIM-1 levels predicted adverse clinical outcomes such as dialysis requirement and mortality. In a previous study, urinary KIM-1 is correlated with AKI severity in non-critically ill children treated by aminoglycosides [12].
Conclusion
Patients treated with aminoglycosides must be carefully monitored for nephrotoxicity. Creatinine has been the most used biochemical marker of AKI, but new biomarkers, such as NGAL and KIM-1, have been developed in recent years.
References
1. Oliveira JF, Silva CA, Barbieri CD, Oliveira GM, Zanetta DM, Burdmann EA. Antimicrob Agents Chemother. 2009; 53(7): 2887-2891.
2. Schmidt-Ott KM. Nephrol Dial Transplant. 2011; 26(3): 762-764.
3. Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. Kidney Int. 2011; 79(1): 33-45.
4. Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. Antimicrob Agents Chemother. 1999; 43(7): 1549-1555.
5. Ricci Z, Cruz DN, Ronco C. Nat Rev Nephrol. 2011; 7(4) :201-208.
6. Delanaye P, Cavalier E, Maillard N, Krzesinski JM, Mariat C, Cristol JP, et al. [Creatinine: past and present]. Annales de Biologie Clinique 2010; 68(5): 531-543 (in French).
7. Waikar SS, Bonventre JV. J Am Soc Nephrol. 2009; 20(3): 672-679.
8. Nguyen MT, Maynard SE, Kimmel PL. Clin J Am Soc Nephrol. 2009; 4(3): 528-34.
9. Delanaye P, Cavalier E, Mariat C, Krzesinski JM, Rule AD. Kidney Int. 2011; 80(5): 439-440.
10. Herget-Rosenthal S, Marggraf G, Husing J, Goring F, Pietruck F, Janssen O, et al. Kidney Int. 2004; 66(3): 1115-1122.
11. Devarajan P. Nephrology (Carlton) 2010; 15(4): 419-428.
12. McWilliam SJ, Antoine DJ, Sabbisetti V, Turner MA, Farragher T, Bonventre JV, et al. PLoS One 2012; 7(8): e43809.
The authors
François Fraissinet1 BSc, Emilie Sacchetto2 and Edith Bigot-Corbel2* PhD
1Laboratoire de Biochimie, 86021 Poitiers, France
2Laboratoire de Biochimie, CHU de Nantes, Hôpital G et R Laënnec, 44800 Saint-Herblain, France
*Corresponding author
E-mail: edith.bigot@chu-nantes.fr