Multiplex specific IgE detection in allergy diagnostics
by Dr Jacqueline Gosink
In vitro determination of specific IgE is nowadays a central pillar of allergy diagnostics. Specific IgE against up to 54 allergens can be investigated in parallel using the EUROLINE immunoblot system. Individual EUROLINE profiles are targeted to specific indications, encompassing food, inhalation, atopy, insect venoms and pediatrics, while diverse region-specific profiles take into account local allergen exposure. Assays based on allergen source-specific components (defined partial allergens) provide in-depth characterization of IgE-mediated sensitizations. Component-resolved profiling allows discrimination of primary sensitizations from cross reactions, as well as differentiation of high-risk and low-risk reactions. This accurate diagnosis is essential for selecting the most suitable immunotherapy and for assessing the risk of severe complications such as anaphylaxis.
Multiparameter immunoblots for allergy diagnostics
In vitro determination of specific IgE antibodies provides a non-invasive method for identifying the triggers of type I allergic reactions. These tests complement conventional allergen provocation methods such as the skin-prick test.
Immunoblots such as the EUROLINE are ideally suited to specific IgE detection, as they enable many different parameters to be investigated simultaneously, yielding extremely detailed patient sensitization profiles. The use of individual membrane chips means that allergens with widely differing properties can be combined on one strip and profiles compiled according to the exact application. Specific IgE antibodies are detected using either whole extracts of the allergen source or defined partial allergens thereof.
Profiles based on allergen extracts
Whole allergen extracts are available for an enormous range of allergen sources and are combined into different profiles focusing on particular applications, such as food allergies, inhalation allergies or atopic reactions. Country- and region-specific EUROLINE profiles are available for e.g. France, the Mediterranean region, Lithuania, Ukraine, the Middle East region, the Gulf states, Lebanon, the Mahgreb, Turkey, India, China, Singapore, Thailand, Mexico, Chile, Peru, and South Africa.
EUROLINE extract-based assays provide fast and comprehensive screening for IgE antibodies against the respective substances. However, cross reactions between allergen extracts may occur due to structural similarities between the proteins present in different sources. For example, cross reactions can occur between different pollens, between pollens and foods, or between different insect venoms.
Profiles based on defined partial allergens
The precise proteins targeted by the specific IgE can be identified using defined partial allergen diagnostics (DPA-Dx). DPA-Dx profiles combine species-specific marker molecules and cross-reactive panallergens in one test, enabling highly differentiated diagnostics for minimal effort. The individual proteins are either purified from the allergen source in native form or produced recombinantly. The defined partial allergens have a high level of standardisation and in some cases a greater stability than is possible with extracts. Moreover, allergenic proteins that are underrepresented in natural extracts but are nevertheless clinically relevant can be included in component-based assays. Comprehensive DPA-Dx profiles are available for foods, insect venoms, pollens and Mediterranean-specific inhalation allergens. The DPA-Dx food allergy tests include a profile for the most common pediatric food allergies, encompassing milk, egg and peanut allergens, and a detailed panel for peanut. A profile for different tree nuts such as hazelnut, walnut and pecan nut is in development.
In particular applications, extracts and defined partial allergens are combined in a single profile. This enables parallel investigation of sensitizations to different whole allergens and precise characterization of important allergic reactions in one test.
Cross-reactive carbohydrate determinants (CCDs)
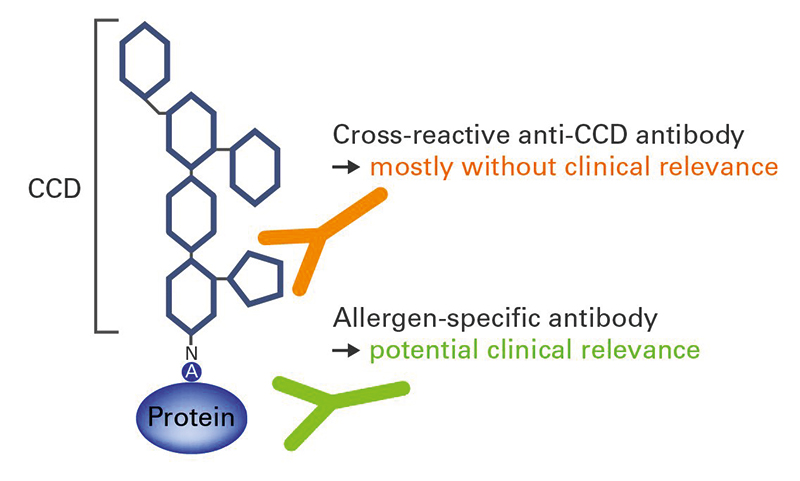
Many allergens are glycoproteins, containing oligosaccharide side chains attached to the protein framework. Some patients develop IgE antibodies against these cross-reactive carbohydrate determinants (CCDs) (Figure 1). Anti-CCD reactions generally have no clinical relevance, but can complicate the interpretation of results in in vitro diagnostics. For this reason, it is helpful to investigate IgE antibodies against CCDs as part of allergy diagnostics to provide additional information on the patient’s sensitization profile. All EUROLINE profiles are equipped with a band of CCD to aid interpretation of positive results. If anti-CCD IgE antibodies are present in patient serum, they can be removed using a special anti-CCD absorbent and the analysis repeated. Anti-CCD antibody reactions are, however, only possible in diagnostics based on particular allergen extracts and native partial allergens; they do not occur with recombinantly-produced components which lack these posttranslational modifications.
The EUROLINE procedure
EUROLINE tests are simple to perform, with a choice of incubation protocols for different requirements. For example, the time-optimized protocol yields results in less than three hours, while the volume-optimized protocol requires only small serum volumes, e.g. 100 µl, making it ideal for use in pediatrics. An indicator band on each strip verifies correct performance of the test.
The immunoblots can be processed manually or fully automatically using the EUROBlotOne (Figure 2). This device provides complete automation of all steps, from sample entry to report release. Up to 44 strips can be incubated per run, and it is possible to combine different profiles in one run. Preanalytical errors, due to wrong positioning of samples, are avoided thanks to the integrated barcode scanner. Moreover, the device automatically takes pictures of the strips with the integrated camera, and evaluates, interprets and archives them with the user-friendly EUROLineScan software. Results are issued in standardized EAST classes.
Example: Insect venom allergies
Insect venom allergies affect around 9 to 29% of the population and are most frequently caused by stings from bees and wasps, less frequently by paper wasps and hornets. In up to 7.5% of sensitized persons they lead to potentially life-threatening systemic reactions. Insect venom allergies can be effectively treated by specific immunotherapy.
In classical diagnostics 50% of persons who are allergic to insect venoms show a sensitization to one venom. The other 50% show reactions to several insect venoms, although only around 17% of patients are actually multiply sensitized. In the remaining 33% cross-reactions are present, which are due to related allergens of the different insect species or unspecific CCDs.
Primary insect venom sensitizations can be reliably differentiated from cross-reactions using the EUROLINE DPA-Dx Insect Venoms 3 profile (Figure 3). The profile contains defined partial allergens from wasp (Vespula vulgaris) and bee (Apis mellifera), complemented by native extracts from wasp, bee and hornet (Vespa crabro). In particular, the profile includes the recombinant component rApi m 10 from bee venom, which is under-represented in native allergen extracts, thus increasing the diagnostic sensitivity for this parameter. The components Api m1 and Api m 10 cover more than 87% of bee venom sensitizations, while Ves v 1 and Ves v 5 encompass over 95% of wasp venom sensitizations. The comprehensive analysis allows discrimination of true double sensitizations from cross reactions and thus provides crucial support for decision-making on suitable specific immunotherapy.
In a comparative study [1], the parameters on the profile showed excellent correlation with single-parameter assays from another commercial supplier, for example, 96% for rApi m 1, 86% for rVes v 1 and 96% for rVes v 5.
Example: Pollen allergies in southern Europe
Inhalation allergies that are common in Mediterranean countries can be differentiated using a newly launched profile which was developed specifically for this region. The EUROLINE DPA-Dx Pollen Southern Europe 1 (Figure 4) provides a unique combination of species-specific markers, cross-reactive components and whole extracts, encompassing tree pollens (birch, olive, cypress, hazel and oak), grass pollen (timothy grass), weed pollen (wall pellitory) and the mould Alternaria alternata. Since the plants represented on the profile have closely overlapping flowering periods, the detailed DPA-Dx analysis provides valuable support in identifying the sensitization source.
The native extracts provide a general screening for IgE antibodies against the respective pollen/mould. The combination of species-specific marker molecules (e.g. nCup a 1, rOle e 1 and rPar j 2) and common panallergens (e.g. PR-10 proteins and profilin) enables precise discrimination between primary sensitizations and cross reactions. Positive reactions with species-specific markers are an indication for specific immunotherapy. Positive reactions with panallergens on the other hand indicate cross-reactions. These molecules are prevalent among different types of plants, which results in a high sensitization rate. An insufficient differentiation between primary sensitizations and cross reactions often results in a less effective desensitization during specific immunotherapy. With this in-depth profile, true sensitizations can be reliably identified, enabling targeted selection of the most suitable specific immunotherapy and improved prognosis of the therapy success.
The high analytical and clinical value of the profile has been verified in an independent study [2]. A further large-scale international multicentre study is currently in progress (www.ait2020.com).
Summary
Allergies pose a serious and increasing burden for patients and healthcare systems globally. Optimal patient management is dependent on accurate identification of the allergy triggers. The EUROLINE system provides comprehensive screening and detailed characterization of specific IgE reactions, and thus provides essential support in allergy diagnostics. In particular, tests based on defined partial allergens can distinguish between primary sensitizations, including multiple sensitizations, and cross reactions. This is essential for clinical decision-making on specific immunotherapy and for advising patients on the risk of life-threatening allergic reactions. Further DPA-Dx profiles for component-resolved analytics in different application areas are in development.
References
1. Micaletto S, et al. Comparison of two methods for measuring IgE to a panel of partly molecular based hymenoptera allergens. EACCI Congress 2016; poster presentation
2. Di Fraia M, et al. A new molecular multiplex IgE assay for the diagnosis of pollen allergy in Mediterranean countries: a validation study. Clin Exp Allergy 2018; Sep 3. doi: 10.1111/cea.13264
The author
Jacqueline Gosink, PhD
EUROIMMUN AG,
Seekamp 31
23560 Lubeck,
Germany