New Code of Practice for stem cell-based embryo models addresses ethical concerns
Scientists in the UK have developed the first code of practice for research using stem cell-based embryo models, providing ethical guidelines and governance for this rapidly advancing field.
In a significant development for stem cell research, a new Code of Practice has been released to guide the use of stem cell-based embryo models (SCBEMs) in scientific studies. This pioneering framework, developed by a multidisciplinary team of experts, aims to address ethical concerns and provide clear boundaries for researchers working with these complex biological structures. Stem cell-based embryo models are three-dimensional structures created in laboratories from stem cells. These models mimic aspects of early human embryo development, offering researchers unprecedented insights into critical stages that were previously inaccessible. The potential applications of this research are far-reaching, with implications for understanding and treating recurrent miscarriage, developmental disorders, and improving in vitro fertilisation success rates.
However, the ability to create structures that mimic human embryos has raised ethical questions and concerns. Until now, there has been no specific regulatory framework for SCBEM research, leaving scientists and research organisations uncertain about the legal and ethical boundaries of their work.
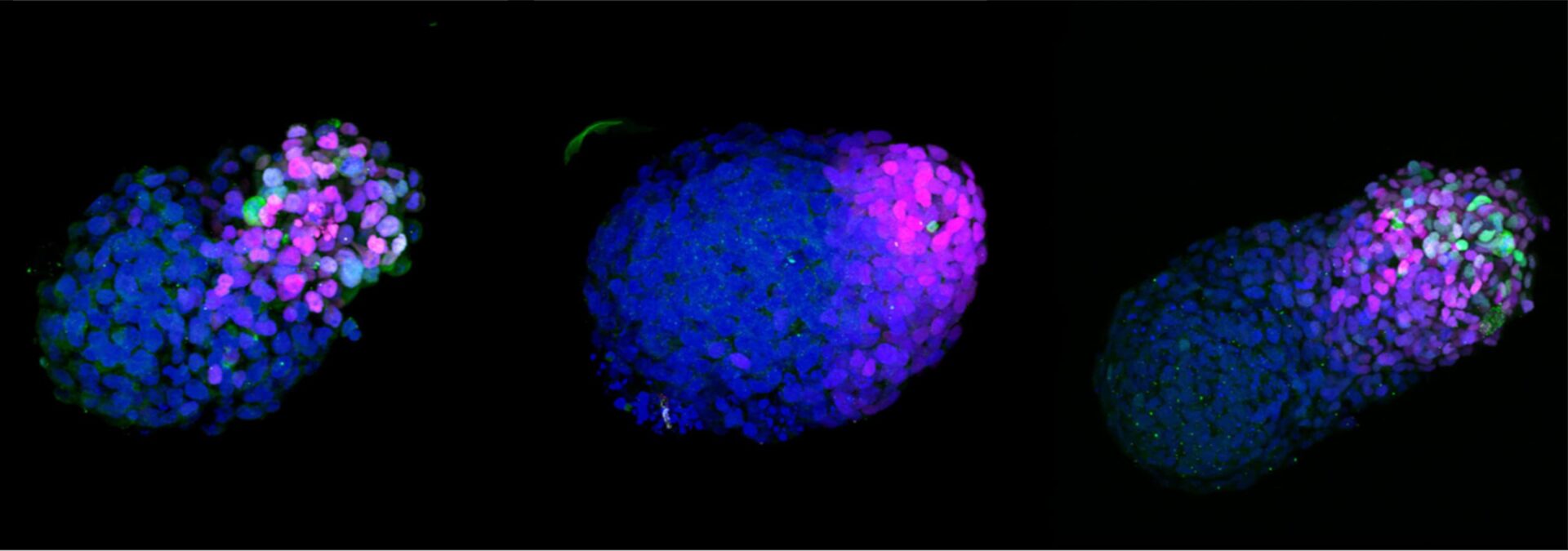
A growing stem cell-based embryo model 24h (left), 48h (middle) and 72h (right) after embryonic stem cell aggregation.
Photo credit: Naomi Moris/University of Cambridge
Key features of the new code
The SCBEM Code of Practice sets out standards to ensure that research using these models is rigorous, ethically sound, and maximises potential benefits. One of the most crucial aspects of the code is the establishment of a dedicated Oversight Committee tasked with reviewing each proposed research project. Professor Kathy Niakan, Chair of Cambridge Reproduction and Professor of Reproductive Physiology at the University of Cambridge, explained: “The new Code of Practice provides processes for decision-making in research using stem cell-based embryo models so that scientists can proceed confidently, while maintaining public trust in this vital area of research.”
Commenting on the new code of practice, Professor Robin Lovell-Badge FRS FMedSci, Group Leader, Francis Crick Institute, said: “Guidelines and Codes of Practice are very useful governance mechanisms, especially for fast moving fields of science. They can be developed, enacted and updated much faster than hard laws, and with buy-in from trusted members of the relevant scientific community they can reassure both researchers, who generally want to work within clear boundaries, and the public that ethical barriers will be respected. Peer pressure, standards set by critical stakeholders, including funders and science publishers, and the risk that a potentially valuable field of research can be damaged or even closed down by laws, all help to reduce the likelihood of something being done by a researcher that crosses boundaries and loses public trust. Of course, there can be maverick or rogue scientists who go too far, but these tend to be individuals who would not even respect hard laws.
“The Code of Practice for the generation and use of stem cellbased embryo models (SCBEMs) that has been developed by the group established by the University of Cambridge and Progress Educational Trust will fill a critical gap in the UK’s regulatory landscape. It is designed to be flexible and to accommodate advances in the field, but through its recommendation to have robust review of projects, it should give reassurance to all interested parties that ethical boundaries will be respected while permitting much valuable research. It adds to the Guidelines published by the International Society for Stem Cell Research in 2021, which include work with SCBEMs (although these are already a little out of date), but in a way that will mesh better with the UK’s way of governing research on human embryology.
“We don’t yet know where the research on SCBEMs will take us, most likely, however, it will be in multiple directions that will give us much better understanding than we currently have of normal human embryo development, of situations where it goes wrong, whether IVF failure, miscarriage, congenital disorders, or adverse effects of drugs and other substances used in pregnant women, and, hopefully of ways to avoid such problems. They will never completely replace the need for using some normal human embryos in research, in part because the models need to be validated by comparing them with the latter, but because they can be generated in large numbers, the SCBEMs will permit types of research that could not be conducted on embryos, such as screens for biologically important drugs or genes.”
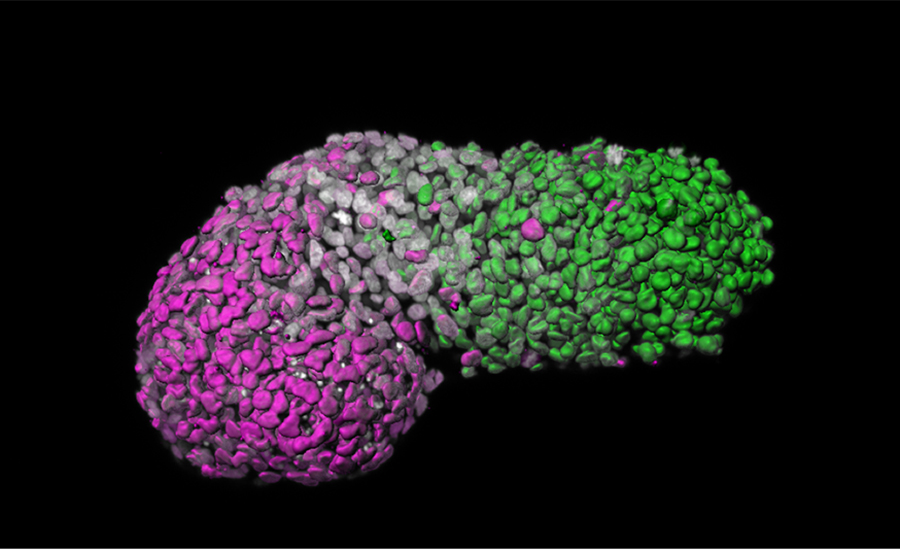
Image analysis of a stem cell-based embryo model showing ‘anteroposterior’ patterning. Green is the posterior part, similar to the tail-end of an embryo; magenta is the anterior part, similar to developing heart cells; grey marks DNA.
Photo credit: Naomi Moris/University of Cambridge
Addressing time limits and ethical boundaries
One of the most challenging aspects of regulating SCBEM research is determining how long these models can be grown in the laboratory. The code recognises the need for limits but acknowledges that a single fixed timeframe is impractical due to the variety of models being developed. Instead, researchers will be required to provide clear justification for the duration of their experiments on a case-by-case basis.
The code also explicitly prohibits the transfer of any human SCBEM into the womb of a human or animal, as well as allowing them to develop into viable organisms in the laboratory. This restriction addresses one of the primary ethical concerns surrounding this type of research.
Potential applications
The use of SCBEMs in research has the potential to significantly advance our understanding of human development, including early pregnancy loss, congenital defects, and factors affecting adult health and disease. These models could also prove invaluable for investigating areas where research on human embryos is not permitted, such as drug safety during pregnancy. Dr Peter Rugg-Gunn, Group Leader and Head of Public Engagement at the Babraham Institute, noted: “Establishing this guidance takes stem cell-based embryo models out of the grey zone and onto more stable footing so we can fully explore their usefulness, while providing the essential reassurance that this research is being conducted carefully and with appropriate scrutiny.”
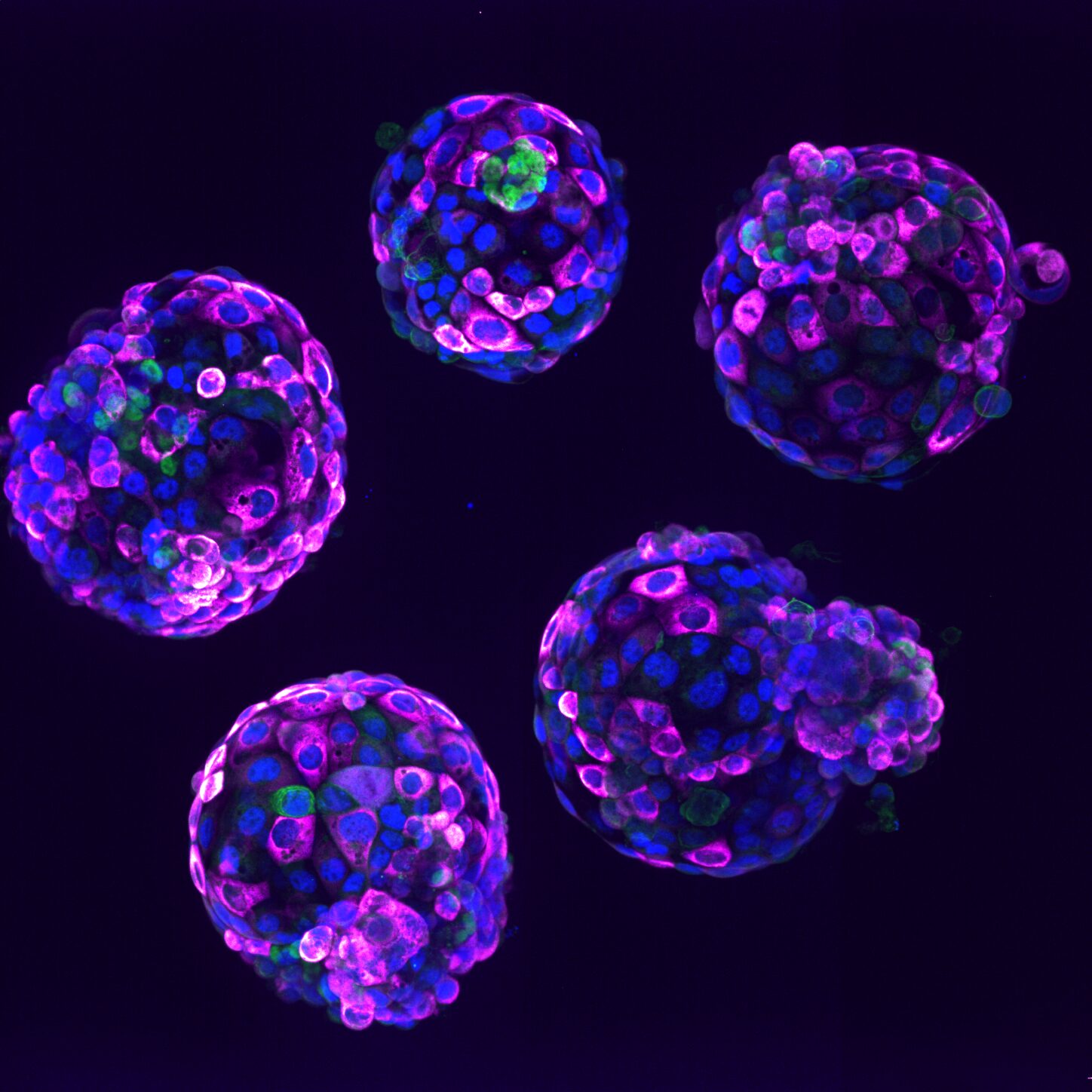
Human stem cell-based embryo model – Blastoids. Blue marks all nuclei, the green label marks cells of the inner cell mass, and the pink label is a readout of a ribosomal protein.
Photo credit: Naomi Moris/University of Cambridge
Development and consultation process
The SCBEM Code of Practice was developed by a working group comprising researchers and practitioners from various institutions across the UK. The team included experts in science, law, ethics, and regulation. To ensure a comprehensive approach, the group consulted widely with researchers, practitioners, funders, and regulators both within the UK and internationally. Importantly, the development process also included a public dialogue to explore public attitudes towards research involving embryo models. Christina Rozeik, Programme Manager of Cambridge Reproduction, highlighted the importance of this engagement: “A public dialogue enabled us to include public voices during the development of the Code, taking account of their hopes, concerns and sensitivities around research involving stem cell-based embryo models.”
Implementation and future reviews
While the code is not legislative, it is expected to be widely adopted by UK researchers, funders, research organisations, professional societies, and publishers. This widespread adoption is anticipated to set a new precedent for reporting research utilising SCBEMs and discourage the funding and publication of research that fails to meet the standards set out in the code. Given the rapidly evolving nature of this field, the code will
be subject to regular review and updates. Professor Niakan emphasised this point, stating: “Research using stem cell-based embryo models is very new, and our understanding of the science is rapidly evolving. We’ve taken the opportunity to respond to the current situation, and we’ll continue to update the Code of Practice as the science continues to develop.”
Download:
Code of Practice for the generation and use of human stem cell-based embryo models: https://t.ly/thl3w