Pediatric reference intervals: tailoring reference intervals to the target population
The use of suitably matched reference values derived from well-characterized individuals is critical to avoid misdiagnosis. Developing reference intervals in pediatric populations presents unique challenges. Recognizing these issues and bridging international biorepository efforts is essential to improving pediatric healthcare.
by Dr Carmen Gherasim, Sonia L. La’ulu, Sara P.Wyness and Dr Joely A. Straseski
Introduction
Children are unique individuals with dynamic developmental physiology which must be accounted for during clinical evaluation. In addition to a medical history and clinical examination, laboratory investigations constitute an integral part of diagnosis and therapeutic management. Understandably, children have limited ability to describe symptoms and medical providers must rely strongly on clinical and laboratory investigations. The availability of pediatric reference intervals (PRI) is, therefore, essential to avoid missed opportunities for treatment of preventable conditions and adverse consequences due to wrong diagnosis.
Depending on the analyte, PRI can differ substantially from RI in adult populations. Marked differences can even be observed within the pediatric population, with profound changes occurring during timeframes such as the first year of life or puberty. The dynamic process of growth, from the initial adaptation of infants living outside the womb to full sexual maturation associated with adulthood, is accompanied by changes in body composition and metabolic, immune, and hormonal fluctuations [1]. Furthermore, developmental stages in children, particularly during puberty, do not always correlate with age and can be affected by factors including nutritional status, body mass index (BMI), medications, or therapies (i.e. growth hormone therapy) [2]. It is therefore advantageous to develop PRI using clearly defined demographics such as age, sex, and race, along with stratifications accounting for physiological and sexual development to assist in clinical decision making.
From a clinical laboratory perspective, the dynamics of the sampled population along with regulatory and administrative requirements for determining PRI pose unique challenges for establishing high quality RI for pediatric populations [3]. Such challenges include: (1) defining ‘healthy’ in children; (2) obtaining research ethics board approval for sample collections; (3) obtaining informed parental and/or child consent for participation; (4) accommodating small sample volumes; and (5) partitioning of data. Collectively, these can hamper access to a sufficient number of specimens from healthy children, precluding many laboratories from performing PRI studies.
Origination of PRI
While RI studies may be performed by in vitro diagnostic manufacturers for commercially available assays, these often lack pediatric data or have minimal stratification reflecting children’s physiological variability. Although not unique to the pediatric population, recycling of RI determined using obsolete methods/instrumentation or adoption of RI from previously published sources without study traceability are often used to report and interpret laboratory data. Caveats in PRI adoption include the limited number of studies performed in pediatric populations, inclusion of data from hospitalized patients, data analysis techniques used, and lack of detailed information from existing studies including population tested and certain patient demographics. Therefore, careful examination of subjects and methods used in existing studies is critical.
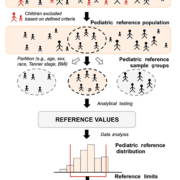
Given the complexity of establishing new PRI, clinical laboratories should carefully consider the option to: (1) transfer, (2) verify or (3) establish PRI based on requirements addressed in guidelines (e.g. Clinical and Laboratory Standards Institute (CLSI) EP28-A3c) [4]. When a laboratory changes analytical methods for measuring an analyte for which they have previously established a RI, that RI may be ‘transferred’ following an acceptable method comparison study. This strategy may prove particularly useful when obtaining pediatric specimens is difficult. Other pre-determined RI must be ‘verified’ before their adoption by examining whether results from a minimum of 20 healthy individuals fall between the proposed limits. Finally, ‘establishing’ new RI requires examining a minimum of 120 samples for each statistically distinct group (partition) selected from qualified, healthy individuals chosen using well-defined inclusion/exclusion criteria (Fig. 1). This is an enormous effort, but particularly onerous in pediatric populations due to limited samples and numerous partitions. Due to this, routine clinical laboratories are often limited to verifying rather than establishing PRI.
Selection of reference populations for PRI
Ideally, reference studies should be conducted using ‘healthy’ volunteers but the identification and recruitment of a healthy representative pediatric population is complex. Health status of recruited children may be assessed by physical examination and health-related questionnaires; however, a definitive delineation of ‘healthy’ is challenging in pediatric populations, as subclinical problems may go unrecognized. Obtaining a sufficient number of pediatric specimens from truly healthy children remains a rate-limiting step of this process.
One strategy that circumvents this challenge is the use of residual blood samples from hospitalized children, deemed healthy due to unrelated or inconsequential medical conditions. This approach eliminates the undesirable blood collection procedure but can be affected by the lack of medical history that can influence test results and overall quality of the PRI established. Additionally, data mining methods exploiting laboratory results from hospitalized children can be used. First introduced by Robert Hoffmann, this approach assumes that the majority of specimens measured in the clinical laboratory represent ‘normal’ values, whereas the most extreme values account for the sickest populations [5].
The effect of common physiological variables such as age and sex on pediatric biomarkers is often evaluated using specific RI partitions. Complex factors such as BMI, Tanner staging, and race may also influence the concentration of select analytes. Despite the rising incidence of pediatric obesity in many developed countries, the effect of BMI on PRIs has been largely overlooked [6]. Challenges in designing studies to address this issue include susceptibility of analytes to BMI variability and delineation between BMI and changes in body composition throughout development. Also, physical and pubertal development in children is not always parallel. Surges in hormone concentrations trigger sexual development and their concentrations vary with the presence of secondary sexual characteristics (described by Tanner stages) rather than age. Finally, race-specific differences can also influence concentrations of pediatric analytes and covariates such as BMI, but their contribution could be underestimated due to their multifactorial etiology.
Statistical methods for PRI analysis
Laboratory data is interpreted in the context of RI, which typically describe the central 95% of results from a population of healthy volunteers. One particular concern with PRI is the availability of sufficient numbers of specimens to allow for statistical significance of each partition [7]. Selection of an appropriate statistical method to compute RI is dependent on the number of specimens in each partition and overall distribution of the data. A small number of pediatric specimens can result in sampling variability which decreases the confidence that a normal result will fall in the established RI. General statistical methods can be employed including parametric (smaller specimen numbers following Gaussian/normal distribution) and nonparametric methods (minimum 120 reference observations per partition). Current CLSI guidelines recommend the use of nonparametric approaches for estimation of RI as they do not make assumptions regarding the distribution of the data [4]. Despite the easy access to data, assumptions regarding data distribution and weak correlation studies often reveal that Hoffmann statistical approaches may be unreliable for establishing new RI in pediatric populations [8]. Increasing the statistical power of PRI determinations and understanding the effect of covariates remains challenging due to the large number of specimens required that can only be reasonably addressed in multicentre RI initiatives.
Current studies emphasizing the need for PRI
The importance of well-defined PRI is highlighted by our recent studies investigating a number of analytes with different concentrations in pediatric populations as compared to adults. A valuable component of our studies is the use of large numbers of well-characterized subjects selected using clearly defined inclusion/exclusion and partition criteria. Whereas some analytes show significant differences between age and sexes (bone markers osteocalcin, procollagen type 1N-terminal propeptide, bone-specific alkaline phosphatase, and C-telopeptide; thyroglobulin and free triiodothyronine), others do not (free thyroxine) [9–11]. PRI for 5α-dihydrotestosterone identified significant differences between sexes and Tanner stages [12]. These differences highlight the need for PRI for individual analytes and the large dataset for each partition in these studies was critical for appropriate data analysis and delineation of proper PRI. As the majority of clinical laboratories verify rather than establish PRI, reported studies should be carefully reviewed for study design, selection of reference individuals, methods used, analytical quality, and appropriate statistical analysis of the data before being considered for PRI adoption.
PRI initiatives
There are a number of initiatives around the world striving to improve the quality and accuracy of PRI (Table 1). Their major advantage is the recruitment of large cohorts of healthy children and adolescents using well-defined selection and partition criteria. Understandably, recruitment strategies vary between initiatives but generally include soliciting members of local communities, organizing clinics at schools and community centres, and/or enrolment prior to undergoing elective, non-invasive, outpatient surgeries. Although each initiative has focused on different biomarkers, most focus on common biochemical markers, blood analytes, vitamins, and/or hormones [13–16]. All studies included partitions for pediatric populations by age and sex and most reference values were analysed using nonparametric statistics to define central 95% PRI. Additional covariates such as Tanner staging, race, or BMI were addressed only by select initiatives including CHILDx, CALIPER and IDEFICS, respectively. A shortcoming of many of the studies was the relatively small representation of other races. Multicentre studies could address this problem, thereby promoting harmonization and diversity amongst PRI. In a recent position statement, the American Association for Clinical Chemistry expressed their support for the foundation of a national repository, enabling a comprehensive evaluation of all PRI covariates and provide and maintain up-to-date PRI databases [17].
Concluding remarks
Reference intervals for pediatric populations weigh heavily in the interpretation of laboratory results and can impact the outcome of clinical decisions. In recent years, we have witnessed an increased awareness of the ‘malpractices’ in PRI including adoption from suboptimal RI studies or from populations that do not mirror the healthy state in children. Recommendations to establish individual PRI that adequately represent the numerous variables in children’s development remain hard to meet by most laboratories, reinforcing a need for a collective effort in establishing PRI. Concentrating national and international efforts can support PRI initiatives and improve pediatric healthcare overall.
References
1. Coffin CM, Hamilton MS, Pysher TJ, Bach P, Ashwood E, Schweiger J, Monahan D, Perry D, Rogers BB, et al. Pediatric laboratory medicine: current challenges and future opportunities. Am J Clin Pathol 2002; 117(5): 683-690.
2. Sikaris KA. Physiology and its importance for reference intervals. Clin Biochem Rev 2014; 35(1): 3-14.
3. Tahmasebi H, Higgins V, Fung AWS, Truong D, White-Al Habeeb NMA, Adeli K. Pediatric reference intervals for biochemical markers: gaps and challenges, recent national initiatives and future perspectives. EJIFCC 2016; 28(1): 43–63.
4. Defining, establishing, and verifying reference intervals in the clinical laboratory. Approved guideline-third edition. CLSI document EP28-A3c. Clinical Laboratory Standards Institute 2008.
5. Hoffmann RG. Statistics in the practice of medicine. JAMA 1963; 185: 864–873.
6. Erhardt E, Foraita R, Pigeot I, Barba G, Veidebaum T, Tornaritis M, Michels N, Eiben G, Ahrens W, et al. Reference values for leptin and adiponectin in children below the age of 10 based on the IDEFICS cohort. Int J Obes (Lond) 2014; 38 Suppl 2: S32–38.
7. Daly CH, Liu X, Grey VL, Hamid JS. A systematic review of statistical methods used in constructing pediatric reference intervals. Clin Biochem 2013; 46(13–14): 1220–1227.
8. Shaw J, Cohen A, Konforte D, Binesh-Marvasti T, Colantonio DA, Adeli K. Validity of establishing pediatric reference intervals based on hospital patient data: a comparison of the modified Hoffmann approach to CALIPER reference intervals obtained in healthy children. Clin Biochem 2014; 47(3): 166–172.
9. Wyness SP, Roberts WL, Straseski JA. Pediatric reference intervals for four serum bone markers using two automated immunoassays. Clin Chim Acta 2013; 415: 169–172.
10. Owen WE, Bunker AM, Straseski JA. Pediatric reference intervals for thyroglobulin using the Beckman Coulter Access 2 immunoassay. Clin Chim Acta 2014; 435: 40–41.
11. La’ulu SL, Rasmussen KJ, Straseski JA. Pediatric reference intervals for free thyroxine and free triiodothyronine by equilibrium dialysis-liquid chromatography-tandem mass spectrometry. J Clin Res Pediatr Endocrinol 2016; 8(1): 26–31.
12. Lin DC, Straseski JA. Tanner stage-stratified pediatric reference intervals for dihydrotestosterone [Abstract]. Clin Chem 2016; 62(10): S188.
13. Colantonio DA, Kyriakopoulou L, Chan MK, Daly CH, Brinc D, Venner AA, Pasic MD, Armbruster D, Adeli K. Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem 2012; 58(5): 854–868.
14. Kohse KP, Thamm M. KiGGS-the German survey on children’s health as data base for reference intervals. Clin Biochem 2011; 44(7): 479.
15. Kant AK, Graubard BI. Race-ethnic, family income, and education differentials in nutritional and lipid biomarkers in US children and adolescents: NHANES 2003-2006. Am J Clin Nutr 2012; 96(3): 601–612.
16. Ridefelt P. Population-based pediatric reference intervals in general clinical chemistry: a Swedish survey. J Med Biochem 2015; 34(1): 64–65.
17. Pediatric lab results: the need for “normal.” AACC Position Statement. AACC 2016; 1–3.
The authors
Carmen Gherasim1 PhD, Sonia L. La’ulu2 BS, Sara P. Wyness2 BA, and Joely A. Straseski*1,2 PhD
1Department of Pathology, University of Utah School of Medicine, Salt Lake City, UT, USA
2ARUP Institute for Clinical and Experimental Pathology, Salt Lake City, UT, USA
*Corresponding author
E-mail: joely.a.straseski@aruplab.com