Serological biomarkers for refractory celiac disease
by Dr Hetty J. Bontkes
Type II refractory celiac disease (RCD-II) and enteropathy associated T-cell lymphoma (EATL) are rare but serious complications of adult onset celiac disease.
These complications are often diagnosed at a late stage. Relatively cheap serological biomarkers could aid in the early identification of RCD-II patients and patients at risk of developing EATL.
Background
Celiac disease (CD) is an autoimmune disease characterized by intolerance to gluten peptides in genetically predisposed individuals, affecting 0.5–1% of the Caucasian population. An autoimmune response leads to enteropathy which eventually results in malabsorption. The typical histological picture of the duodenum in CD is described by the modified Marsh criteria, ranging from intraepithelial lymphocytosis to complete loss of duodenal villi, i.e. total villous atrophy. The only successful treatment of CD is a strict lifelong gluten-free diet (GFD). Upon depletion of gluten from the diet mucosal recovery occurs in the majority of patients within 5 years. Although CD previously was regarded as a childhood disorder, CD is now more and more diagnosed in adults. In part of these adult onset CD patients, mucosal damage does not recover, or reoccurs despite long-term strict adherence to a GFD [1]. After excluding unnoticed gluten intake and other causes of villous atrophy these patients are diagnosed with refractory celiac disease (RCD) [2].
Refractory celiac disease
Two types of RCD have been described, depending on the population of intraepithelial T-lymphocytes (IEL) with an aberrant phenotype, i.e. lacking CD3 on the cell surface. In RCD-I these aberrant IEL are present at low frequencies (20%) and frequently transforms into an aggressive enteropathy associated T-cell lymphoma (EATL) [3]. RCD-II can therefore be defined as an indolent lymphoma. Apart from the increased risk of developing EATL, RCD-II is a serious condition characterized by persistent diarrhea, abdominal pain, anemia, hypoalbuminemia, vitamin deficiencies, and cachexia [4]. RCD-II is treated with immunosuppressive agents or the chemotherapeutic agent cladribine. Although cladribine induces clinical and histologic improvement in the majority of cases, about 50% of RCD-II patients will nevertheless develop EATL (3). Autologous stem cell transplantation (ASCT) following cladribine therapy has been shown to be effective, but unfortunately many patients are not eligible for ASCT due to their physical condition at the time of diagnosis and/or age [5].
Enteropathy associated T-cell lymphoma
EATL is a rare extra-nodular T-cell non Hodgkin lymphoma originating from intestinal intraepithelial T-lymphocytes. Two types of EATL are defined by the WHO; type I comprises 80–90% of the EATL and is in contrast to type II EATL associated with CD(6). In addition to EATL arising from RCD-II, a substantial proportion of type I EATLs develop without a RCD-II stage. EATL typically occurs later in life (sixth or seventh decade) and is more common in men than women, even though incidence of CD is higher in women. Treatment currently consists of resection in combination with high dose chemotherapy with ASCT (7). Despite this aggressive therapy, the 5-year survival is extremely low (5%) and most patients die from this devastating disease within the first year. As even very aggressive therapy is not effective, it is of the utmost importance to identify patients at risk before the EATL develops. The association of the majority of cases with CD provides a unique window of opportunity for the identification of individuals that are at risk and prevention of EATL development by offering these at-risk patients more intensive therapy. Early identification of RCD-II may also allow more intensive therapy, such as ASCT, and should prevent EATL development. The development of biomarkers that predict the development of RCD-II and EATL in CD patients is particularly urgent in the light of rising incidence of adult onset CD and an ever increasing life expectancy in Western Europe and thus an expected increase in EATL incidence. Such biomarkers will have to be determined by low-invasive and relatively cheap tests as all adult-onset CD patients on a GFD should be screened at regular intervals. Serological tests comply with these requirements.
Biomarkers for CD and RCD
Determination of autoantibodies against the enzyme tissue transglutaminase (TGA) plays an important role in the diagnosis of active CD and the follow-up of patients on a GFD. As the generation of these autoantibodies is dependent on the presence of gluten peptides, TGA will decline when gluten is eliminated from the diet. Regular determination of anti-TGA antibody titres is therefore used to monitor compliance to the diet (8). Consequently, these antibodies are less useful in diagnosis and follow-up of RCD patients since these patients have been on a GFD for at least a year and are negative for these antibodies. Therefore, other serological markers are needed in addition to TGA, which are independent of gluten intake and specifically indicate mucosal damage. Intestinal fatty-acid binding protein (I-FABP) is a protein that is located in the enterocytes of the small intestine. It is believed that upon enterocyte damage I-FABP is released into the blood stream. Indeed (I-FABP) levels are related to intestinal damage in uncomplicated CD (9). Actin IgA antibody (AAA) titres are associated with the severity of intestinal damage in active CD as well [10]; whether I-FABP levels and/or AAA titres increase again in RCD is currently under investigation.
Alternatively, antibodies against luminal antigens are promising in this respect. Due to the intestinal damage, there is an enhanced intestinal permeability which may lead to increased exposure of the immune system to luminal antigens in the lamina propria and therefore more antibodies against these antigens are generated. Indeed, approximately 30–80% of adult active CD patients are positive for IgA antibodies against the baker’s yeast (Saccharomyces cerevisiae; ASCA) or antibodies against bovine serum albumine (ABSA). More recently IgA antibodies against another luminal protein, the pancreatic glycoprotein-2 (GP2A), have been shown in patients with active CD [11].
Biomarkers under investigation
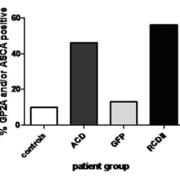
We investigated whether ASCA, ABSA and GP2A are predictive for intestinal damage and/or RCD-II. In a group of uncomplicated CD, ASCA levels decreased together with TGA titres [12]. In subsequent studies we analysed ASCA, ABSA and auto-antibody levels to the luminal antigen GP-2 (GP2A) in relation to GFD, intestinal damage, and RCD-II diagnosis and follow-up. Before treatment a wide range of ABSA, ASCA and GP2A levels was found in both CD and RCD-II patients. Within the active CD group 45.7% had ABSA, 31.4% had ASCA and 28.9% had GP2A levels above the cut-off, in the RCD-II group this was 60%, 50% and 33.3% respectively. The mean levels of all three markers were significantly higher in CD and RCD-II patients compared to controls. In uncomplicated CD, levels of all three markers declined upon a GFD, but in RCD-II patients successful treatment was not predicted by a decline in antibody titres. Interestingly, ASCA and GP2A levels were able to distinguish RCD patients from GFD patients [receiver operating characteristic (ROC) area under curve (AUC)=0.74; P=0.02 and AUC=0.79, P=0.002 respectively). When both tests were performed and at least one test was positive at a cut-off with a specificity of 87%, the sensitivity was 56% (AUC=0.83, P=0.05 and Fig. 1) [13, 14]. Whether these markers are also increased in patients who developed an EATL with or without a previously diagnosed RCD-II stage is currently under investigation. In conclusion: ASCA and GP2A are potential serological biomarkers for the prediction of RCD-II in adult-onset CD patients on a GFD. However, identification of additional serological biomarkers is necessary to increase the sensitivity of these tests.
References
1. Hutchinson JM, West NP, Robins GG, Howdle PD. Long-term histological follow-up of people with coeliac disease in a UK teaching hospital. QJM. 2010; 103: 511–517.
2. Leffler DA, Dennis M, Hyett B, et al. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol. 2007; 5: 445–450.
3. Al-Toma A, Verbeek WH, Hadithi M, et al. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut 2007; 56: 1373–1378.
4. Nijeboer P, van Wanrooij RL, Tack GJ, et al. Update on the diagnosis and management of refractory coeliac disease. Gastroenterol Res Pract. 2013; 2013: 518483.
5. Al-toma A, Visser OJ, van Roessel HM, et al. Autologous hematopoietic stem cell transplantation in refractory celiac disease with aberrant T cells. Blood 2007; 109: 2243–2249.
6. Cellier C, Delabesse E, Helmer C, et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet 2000; 356: 203–208.
7. Ferreri AJ, Zinzani PL, Govi S, Pileri SA. Enteropathy-associated T-cell lymphoma. Crit Rev Oncol Hematol. 2011; 79: 84–90.
8. Leffler DA Schuppan D. Update on serologic testing in celiac disease. Am J Gastroenterol. 2010; 105: 2520–2524.
9. Adriaanse MP, Tack GJ, Passos VL, et al. Serum I-FABP as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibodies. Aliment Pharmacol Ther. 2013; 37: 482–490.
10. Granito A, Muratori P, Cassani F, et al. Anti-actin IgA antibodies in severe coeliac disease. Clin Exp Immunol. 2004; 137: 386–392.
11. Bonaci-Nikolic B, Spuran M, Andrejevic S, Nikolic M. Autoantibodies to GP2, the major zymogen granule membrane glycoprotein, in patients with gluten-sensitive enteropathy: a possible serological trap. Clin Chim Acta. 2012; 413: 822–823.
12. Mallant-Hent RC, Mary B, von Blomberg E, et al. Disappearance of anti-Saccharomyces cerevisiae antibodies in coeliac disease during a gluten-free diet. Eur J Gastroenterol Hepatol. 2006; 18: 75–78.
13. Gross S, van Wanrooij RL, Tack GJ, et al. Antibody titers against food antigens decrease upon a gluten-free diet, but are not useful for the follow-up of (refractory) celiac disease. Eur J Gastroenterol Hepatol. 2013; 25: 516–518.
14. Gross S, Bakker SF, van Bodegraven AA, et al. Increased IgA Glycoprotein-2 specific antibody titres in refractory CD. J Gastrointestin Liver Dis. 2014; 23: 127–133.
The author
Hetty Bontkes PhD
VU University Medical Center, Department of Pathology,
Unit of Medical Immunology, Amsterdam, The Netherlands
E-mail: hj.bontkes@vumc.nl