Teicoplanin in therapeutic drug monitoring
Recent years have seen the emergence of teicoplanin usage for staphylococcal infections, in particular endocarditis, osteomyelitis, septic arthritis, and methicillin-resistant Staphylococcus aureus (MRSA). Teicoplanin is now routinely used as an alternative to vancomycin, due to its safety profile and ease of administration. This article discusses the advantages for teicoplanin, the need for routine monitoring, and the various associated methodologies.
by Dr Francis H. Y. Fung
Background
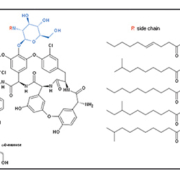
Teicoplanin was first isolated and identified from antibiotic-producing strains of Actinoplanes teichomyceticus in 1978, and was shown to be highly active against both aerobic and anaerobic Gram-positive pathogenic bacteria [1]. This anti-bacterial agent was initially thought to belong in the class of glycopeptide antibiotics that act as cell wall inhibitors such as vancomycin, ristomycin, and mannopeptins. Barna et al. [2] first described the molecular structure of teicoplanin A2 in 1984, as five similar glycopeptides characterized by different fatty acid chains of 10 to 11 carbon atoms (which make up the majority of teicoplanins found in vivo) (Fig. 1), and four minor related compounds that may also be present in very minute quantities. The five major side chains (A2-1 to A2-5) have terminal groups of CH–NH2, whereas in the four minor compounds (R2-1 to R2-4) this is substituted by a C=O group.
The letter ‘R’ denotes where each fatty acid chain attaches to the side group on the teicoplanin molecule. Its structure contains the identical ABCD ring system that is also found in vancomycin, another antibiotic within the same family. In addition to a small modification in the DE ring system, teicoplanin lacks the β-hydroxyl group, a site of sensitivity in vancomycin, and it also has an additional FG ring system that is not present in vancomycin. It is these subtle yet crucial differences that will prove to be a major differentiating factor between the two drugs.
Teicoplanin versus vancomycin
In vitro binding studies revealed that teicoplanin interferes with the final stage of peptidoglycan synthesis (glycan polymerization and cross-linking) by binding to the terminal amino N-acyl-D-alanyl–D-alanine of the growing peptidoglycan or its precursors [2]. This N-acyl-D-alanyl–D-alanine group binds and forms a stoichiometric complex via the formation of five hydrogen bonds between the peptide backbone of the glycopeptide and the D-Ala–D-Ala dipeptide. The formation of this complex prevents any further peptidoglycan synthesis by sterically hindering the correct alignment of transglycosylase and its substrate. The antibiotic activity and potency of teicoplanin, particularly against Streptococcus and Enterococcus species, are also well documented [3]. Despite the increasing frequency of glycopeptide resistance, teicoplanin has always shown clinical worth, especially in treatment of life-threatening sepsis.
Vancomycin, a similar antibiotic in the same glycopeptide family, has historically been the drug of choice for the treatment of diseases caused by methicillin-resistant Staphylococcus aureus (MRSA). The emergence of MRSA strains in the community setting can cause infections ranging from cellulitis with skin abscesses to pneumonia in otherwise healthy individuals. Although vancomycin hydrochloride has been the accepted standard therapy for MRSA infections, its potential nephrotoxicity is one of the major limitations for its routine use. Some studies have found an increased risk of renal failure following vancomycin treatment [4], because of its effects on proximal tubular cells where the antibiotic can accumulate inside lysosomes.
Teicoplanin has been shown to have essentially the same efficacy as vancomycin but with some advantages, such as once-daily bolus administration for dose management. In addition, the pharmacokinetic profile of teicoplanin also offers the patient a choice of administration routes of either intramuscular or intravascular delivery. Several side effects of vancomycin treatment are known, such as ‘red man’ syndrome, a combination of erythema, pruritus, and hypotension, which can be an immediate adverse event of vancomycin infusion. The likelihood of this reaction is greatly reduced with teicoplanin [5]. Perhaps the most widely recognized clinical problem with the vancomycin regime is nephrotoxicity in the patient. Meta-analysis of comparative trials have demonstrated that adverse events were less likely to occur with teicoplanin (13.9%) compared with vancomycin (21.9%) (P=0.0003). This was particularly significant when nephrotoxicity was considered: 4.8% versus 10.7% (P=0.0005) for teicoplanin and vancomycin, respectively [6]. This is of added relevance for patients with renal failure, as evidence suggests dosage adjustment is of paramount importance because of their impaired renal clearance.
A number of studies have been carried out comparing the efficacy and safety of these two drugs, showing the potential for teicoplanin as a suitable alternative to vancomycin [7]. This systematic review and meta-analysis of 24 randomized controlled trials in 2009 highlighted the advantages of teicoplanin over vancomycin. More importantly, total adverse events were found to be less frequent, and nephrotoxicity to be lower for teicoplanin usage, especially when administered in combination with aminoglycosides. The safety profile of teicoplanin has also been shown to be favourable, where severe skin reactions can result due to vancomycin-related infusion and the subsequent release of histamine [8]. Comparison studies with other drugs also used for bacterial infection treatments such as clindamycin, rifampicin, netilmicin, and enoxacin provide further support for the pharmacological profile of teicoplanin [9].
There is evidence of disadvantages in teicoplanin administration, such as hypersensitivity manifesting as fever and chills. Some patients with a positive reaction to teicoplanin can also tolerate vancomycin, where no major advantage lies with either drug. Thrombocytopenia can also develop, although this occurred almost exclusively in patients receiving much larger teicoplanin doses than are now recommended. The majority of the available evidence favours the bacteriolytic effect of teicoplanin [10], where base concentrations of just 1 µg/mL can cause rapid lysis of streptococcal cells in exponential phase. Due to increased bacterial resistance to vancomycin through the substitution of the D-Ala–D-Ala terminus of the peptidoglycan precursor by D-Ala–D-Lac, teicoplanin does seem to hold several major advantages. In addition to the similar and competitive cost of treatment, teicoplanin is emerging as a suitable and appropriate alternative to vancomycin for Staphylococcus infections.
Therapeutic drug monitoring
Therapeutic drug monitoring (TDM) plays an important role in the optimization of drug therapy, especially for drugs with narrow therapeutic ranges. Since the introduction of home therapy and the use of teicoplanin in the community, clinicians have paid close attention to serum concentrations of teicoplanin in their administration regimen, as multifactorial education programmes with active TDM have been shown to be efficacious in maintaining the appropriate use of antibiotics in various healthcare settings [11].
Predominately (>90%) bound to plasma proteins, teicoplanin is mostly cleared renally and only 2–3% of an intravenously administered dose is metabolized. Total teicoplanin clearance rate has been reported to be 11 mL/h/kg, and steady state is achieved slowly – 93% after 14 days of repeated administration [12]. It is believed an optimal loading dose followed by appropriate maintenance doses should achieve the teicoplanin trough serum concentration of 25 µg/mL rapidly and steadily, increasing the chances of full recovery for the patient. Care should be taken when teicoplanin is substituted for vancomycin, as it has a longer half-life: about 40 hours compared to that of 3–5 hours for vancomycin [13]. Because of its longer half-life, teicoplanin only needs to be administered once daily. Administration should be tailored to the individual, as each patient has varying degrees of hepatic and renal function: age, infection, and concomitant use of certain drugs are a few factors that can alter the rate of clearance. Nevertheless, an 800 mg initial dose followed by 400 mg maintenance doses for the following 48 hours has been accepted for safely achieving an optimal teicoplanin trough level. Re-assessment would then take place to determine if additional maintenance doses are needed, and, if so, what the course of action would be. It is important to ascertain the ideal pre-dose serum concentrations, as it can be a predictor of outcome for the patient. However, the rate of success with the treatment declines with age, again highlighting the need to consider other factors pertaining to the individual patient.
Measurement of teicoplanin: a brief history
The solid-phase enzyme receptor assay (SPERA) was most widely used for TDM initially, capable of measuring glycopeptides of the vancomycin family. The assay was based around the interaction of teicoplanin and acyl-D-alanyl–D-alanine, where the antibiotic of interest and enzyme-labelled teicoplanin compete for a synthetic analogue of the biological receptor, albumin-E-aminocaproyl-D-alanyl–D-alanine. Using this method for teicoplanin determination in human serum the intra-assay CV (coefficient of variation) was reported to be 7.2%, inter-assay CV to be 11.2%, and the analytical recovery to be 94% [14]. SPERA correlated well with other available microbiological assays at the time, and it was accepted as a good tool for identification and quantitative detection of teicoplanin.
The disk diffusion (or Kirby Bauer) test used Petri dishes containing the antimicrobial agent covering the surface of the agar. The size of the area free of the microbe being tested was proportional to the effectiveness of the antibiotic. Similarly, the agar incorporation method used viable colonies from overnight cultures to inoculate Sensitivity Test Agar plates containing doubling teicoplanin concentrations. The lowest concentration that inhibited growth would then be assigned the MIC (minimum inhibitory concentration) value. These were more of a qualitative rather than a quantitative assay, and its clinical usage was fairly limited. There were also commercially available kits, such as the high-inoculum Etest method (AB Biodisk, Solna, Sweden) that detects intermediate glycopeptide susceptibility. Etest strips covered with teicoplanin are exposed to infusion agar and incubated for 48 hours at 37°C, and results read at the point of complete growth inhibition. A VITEK assay from bioMerieux (Marcy l’Etoile, France) also used overnight agar plate cultures to measure teicoplanin effect and determined its MIC. This latter method could be automated for the clinical laboratory; however, it still lacked the precision one expects from an assay used for TDM in MRSA resistance.
Increasing popularity of immunoassays paved the way for the implementation of fluorescence polarization immunoassay (FPIA). Based on the principle of competitive binding and the reagent limited concept, the TEICOPLANIN Assay System (Abbot TDx) uses fluorescein-labelled antigen to compete with sample antigen for a fixed number of antibody binding sites. In recent years there has been an emergence of high-performance liquid chromatography (HPLC) as an alternative to FPIA, a method that has several advantages over the traditional immunoassay. The improvements lie within the various components that constitute an HPLC system, since the characteristics of various compounds in a sample matrix can be very different (Fig. 2). Tandem mass spectrometry assays have also recently been developed to measure teicoplanin in patient serum [15], providing the most sensitive method yet for monitoring this therapeutic drug.
Conclusion
As teicoplanin administration gains popularity with clinicians dealing with TDM, there lies a growing need for close assessment of its serum levels. In addition to clinical outcomes, accurate measurement of teicoplanin allows the administration of a definitive loading dose and appropriate maintenance doses to ensure the rapid recovery of patients and improved quality of life. A large number of assays are now available in clinical biochemistry laboratories, and end users are encouraged to adopt these methodologies.
References
1. Parenti F, Beretta G, Berti M, Arioli V. Teichomycins, new antibiotics from Actinoplanes teichomyceticus Nov. Sp. I. Description of the producer strain, fermentation studies and biological properties. J Antibiot. (Tokyo) 1978; 31: 276–283.
2. Barna JC, Williams DH. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu Rev Microbiol. 1984; 38: 339–357.
3. Parenti F, Schito GC, Courvalin P. Teicoplanin Chemistry and Microbiology. J Chemother. 2000; 12(Suppl 5): 5–14.
4. Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006; 166: 2138–2144.
5. Wilson AP. Comparative safety of teicoplanin and vancomycin. Int J Antimicrob Agents 1998; 10: 143–152.
6. Wood MJ. Comparative safety of teicoplanin and vancomycin. J Chemother. 2000; 12(Suppl 5): 21–25.
7. Svetitsky S, Leibovici L, Paul M. Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis. Antimicrob Agents Chemother. 2009; 53: 4069–4079.
8. Davey PG, Williams AH. A review of the safety profile of teicoplanin. J Antimicrob Chemother. 1991; 27(Suppl B): 69–73.
9. Covelli I, Nani E. Microbiological profile of teicoplanin. J Chemother. 1991; 3(Suppl 1): 39–42.
10. Chmara H, Ripa S, Mignini F, Borowski E. Bacteriolytic effect of teicoplanin. J Gen Microbiol. 1991; 137: 913–919.
11. Pea F, Viale P, Pavan F, Tavio M, et al. The effect of multifactorial, multidisciplinary educational interventions on appropriate use of teicoplanin. Int J Antimicrob Agents 2006; 27: 344–350.
12. Wilson AP. Clinical pharmacokinetics of teicoplanin. Clin Pharmacokinet. 2000; 39: 167–183.
13. Boger DL. Vancomycin, teicoplanin, and ramoplanin: synthetic and mechanistic studies. Med Res Rev. 2001; 21: 356–381.
14. Corti A, Rurali C, Borghi A, Cassani G. Solid-phase enzyme-receptor assay (SPERA): a competitive-binding assay for glycopeptide antibiotics of the vancomycin class. Clin Chem. 1985; 31: 1606–1610.
15. Fung FH, Tang JC, Hopkins JP, Dutton JJ, et al. Measurement of teicoplanin by liquid chromatography-tandem mass spectrometry: development of a novel method. Ann Clin Biochem. 2012; 49(Pt 5): 475–481.
The author
Francis Fung PhD
Department of Clinical Biochemistry, Royal Liverpool University Hospitals, Liverpool, UK
E-mail: francis.fung@nhs.net