Testing for gastrointestinal cytomegalovirus infection
by Dr Jingmei Lin and Dr Rong Fan
Cytomegalovirus infection in the gastrointestinal tract likely occurs in periods of immunosuppression. The diagnosis of cytomegalovirus infection in the gastrointestinal tract can be made in several ways including serology, testing for viremia, viral culture from biopsy material, histopathologic examination coupled with immunohistochemistry, and molecular method utilizing quantitative real-time polymerase chain reaction.
Background
Cytomegalovirus (CMV), a member of the herpesvirus family, exists commonly in the healthy population. Similar to other herpesviruses, CMV maintains the ability to establish persistent or latent infection in its host [1]. CMV disease or reactivation may occur during periods of stress or other causes of immunocompromise/suppression (e.g. organ/stem cell transplants, chemotherapy treatment, HIV infection, recipients of immunosuppressive drugs, etc), resulting in systemic disease or end organ involvement.
Patients may present asymptomatic viremia or CMV disease. The latter denotes the presence of CMV infection that is accompanied by clinical symptoms, signs or evidence of tissue involvement. CMV disease can be further categorized into CMV syndrome and CMV tissue-invasive disease. Among the latter, the gastrointestinal track is the most common manifestation, up to 25% of cases [2, 3]. CMV disease can occur at any location in the gastrointestinal tract, from mouth to rectum. One study found that upper gastrointestinal tract involvement was more common in patients with hematological malignancies or stem cell transplantation; while colorectal involvement was more common in patients with solid tumours [4].
Clinical presentation
The clinical presentation of patients with gastrointestinal CMV disease varies depending on the affected organ. Patients with CMV esophagitis may present with odynophagia and constant substernal pain. Epigastric pain and nausea are the classic symptoms of CMV gastritis. Patients with colorectal CMV disease may develop diarrhea, hematochezia, urgency, tenesmus, and abdominal pain. However, none of these symptoms is specific given the overlapping with other diseases, such as graft versus host disease, rejection or other infectious etiologies.
Diagnosis
The diagnosis of CMV infection in the gastrointestinal tract can be suggested by multiple methods including serology, testing for viremia, viral culture from biopsy material, histopathologic examination coupled with immunohistochemistry, as well as recent reports of molecular methods utilizing quantitative real-time polymerase chain reaction (qRT-PCR).
Serology testing
Serology testing, which reflects prior CMV exposure, was once frequently used and is of limited use nowadays due to the prevalence of the infection in the population [5]. However, in immunocompromised hosts, serology is still helpful in determining whether patients are at risk of acquiring a new CMV infection (seronegative) or reactivation (seropositive). Of note, seropositivity does not preclude the possibility of a new infection with an antigenically dissimilar CMV virus strain. Serology specific for IgM antibody is useful when there is a concern for acute infection, although IgM levels may persist for months following exposure [6]. Confounders of CMV serology testing include low immunoglobulin states, plasmapheresis, and transfusion of blood products.
Viremia assays
Viremia assays that test for either antigen or nucleic acid in leukocytes, whole blood, or plasma specimens have become clinically useful. CMV lower-matrix protein pp65 antigenemia is a semi-quantitative test that is relatively easy to perform and does not require expensive equipment, although a lack of assay standardization results in subjective interpretation. The test is limited in patients with neutropenia and the protein is of short stability in blood specimens (testing is required within 6–8 hours of collection) [7]. Currently, quantitative nucleic acid assay is the essential option in laboratory diagnostics. This technique provides not only the most sensitive method to detect CMV in blood but also provides a quantitative measurement for disease progression and treatment effect [8-10]. However, the test requires expensive equipment and specialized expertise.
CMV culture
CMV culture was once the ‘gold standard’ for diagnosis from many body sites, including the gastrointestinal tract. Traditional culture is slow, expensive, and less sensitive in that it requires a lengthy time period (1–6 weeks) in order to observe the typical cytopathic effects on fibroblast monolayer. The advent of early antigen detection by shell vial culture has shortened the time to 2–3 days and its use has become standard [11]. Culture of gastrointestinal tissue samples remains an important resource for diagnosis of tissue-invasive disease, where viremia or qRT-PCR testing on peripheral blood may not always be positive in localized diseases of gastrointestinal CMV infection.
Histopathology
The presence of CMV in the gastrointestinal-tract tissue specimen is necessary for the diagnosis of localized CMV infection. However, histopathology requires an invasive procedure to obtain tissue, which might limit its use in certain clinical settings. For instance, a gastrointestinal biopsy might not be performed if the patient appears to have CMV infection in the gastrointestinal tract with compatible symptoms as well as high levels of CMV detected in blood. However, biopsy is warranted if other critical differential diagnoses exist (i.e. rejection), localized disease without detectable virus in blood, or suspicion of coinfection with other pathogens, especially when anti-CMV treatment does not resolve clinical symptoms.
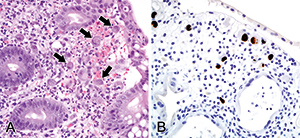
Identification of CMV infection by hematoxylin and eosin (H&E) stained tissue sections relies on the presence of classic viral inclusions (Fig. 1A). However, there are occasions when viral inclusions are not apparent, but clinical suspicion of CMV infection is high, and in these circumstances immunohistochemistry for CMV is frequently employed to enhance diagnostic sensitivity (Fig. 1B). Immunohistochemistry also has its drawback and can be hampered by rare atypical staining patterns, leading to equivocal interpretations. It is a sensitive means of detection, and the clinical significance of a single CMV immunohistochemistry-positive cell is debatable – a consensus has not yet been reached as to what extent detection of CMV in biopsies should be considered clinically significant. For example, is a single immunohistochemistry-positive cell sufficient for the diagnosis of CMV colitis?
Molecular methods
Methods of CMV detection have evolved rapidly as medicine continuously demands faster and more accurate diagnoses. Therefore, molecular methods to detect CMV in gastrointestinal biopsies as an adjunct to H&E and immunohistochemistry have been employed [12-14]. qRT-PCR is a rapid, sensitive and specific method for detecting CMV in formalin-fixed, paraffin-embedded gastrointestinal biopsy tissues compared to traditional histology or immunohistochemistry techniques [13]. In a study using PCR to detect CMV infection in ulcerative colitis patients, CMV DNA was detected in 57% of patients with severe immunosuppressive/resistant disease. Of these patients, 70% were treated with ganciclovir and the majority (83%) of them achieved remission. In contrast, 93% of the CMV-DNA-negative patients achieved remission with immunosuppressive therapy alone [12].
As a new method, the exact role of utility and the clinical significance of qRT-PCR testing are still being defined. Hitherto, there is no consensus on the criteria of diagnosing CMV gastrointestinal disease based on molecular testing results alone. The facts to be pondered are: (1) the high sensitivity of PCR testing leads to the detection of CMV virus in the absence of clinical symptoms or histological evidence of tissue damage; which might represent a remote infection or a low level replication of CMV with uncertain clinical significance. (2) It is unknown what level of viral DNA in gastrointestinal tissue is sufficient to diagnose CMV infection in tissue. (3) Testing results are sometimes not consistent. Is a positive CMV PCR result with negative histology sufficient for a diagnosis?
The set of criteria required for diagnosis may have to be dictated by the clinical circumstances. It is critical to evaluate the significance of detectable CMV in correlation with the patient’s symptoms. High viral load in gastrointestinal tissue is likely to point towards a CMV disease when concurrent appropriate signs and symptoms are present clinically. In other words, if the patients have diarrhea or other gastrointestinal related symptoms with the detection of CMV via biopsy, either on H&E alone or consolidated by immunohistochemistry or PCR, current CMV infection is considered highly likely to be the cause of disease unless other etiologies are evident. On the other hand, when a minimal CMV presence is suggested by immunohistochemistry-stained foci or PCR-positive results alone in an asymptomatic patient, the possibility of a latent viral status needs to be entertained and careful clinical follow-up is warranted. Finally, it is worth pointing out that due to its high sensitivity, the negative predictive value of PCR is high, so it can be very useful to rule out CMV disease.
Conclusions
Early diagnosis and treatment of CMV infection in immunocompromised patients reduces morbidity and mortality significantly, with much improved clinical outcomes. At the current stage of laboratory science, diagnosis of most CMV infections can be rendered easily by histology with the occasional aid of immunohistochemistry; qRT-PCR is an especially helpful tool in ambiguous cases when the above conventional methods fail to establish a conclusive diagnosis. It is also highly enlightening for cases where clinical suspicion for CMV infection runs against available evidence; it also helps in cases where the pathologist finds H&E is suspicious but immunohistochemistry is negative. Finally, the way to incorporate this sensitive CMV detection method in various clinical settings is suggested in the algorithm shown in Figure 2.
Acknowledgements
We acknowledge Tracey Bender and Amy Thomasson for their assistance in preparing this manuscript, and Fredrik Skarstedt for his effort in the preparation of figures.
References
1. Hodinka RL. Human cytomegalovirus. In: Versalovic JC, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW. (ed.) Manual of clinical microbiology. ASM Press 2011, pp. 1558–1574.
2. Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L, Young JA, Rodriguez T, Maertens J, Schmitt M, Einsele H, Ferrant A, Lipton JH, Villano SA, Chen H, Boeckh M. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis. 2011; 11(4): 284–292.
3. Humar A. Reactivation of viruses in solid organ transplant patients receiving cytomegalovirus prophylaxis. Transplantation 2006; 82(2 Suppl): S9–S14.
4. Torres HA, Kontoyiannis DP, Bodey GP, Adachi JA, Luna MA, Tarrand JJ, Nogueras GM, Raad II, Chemaly RF. Gastrointestinal cytomegalovirus disease in patients with cancer: a two decade experience in a tertiary care cancer center. Eur J Cancer 2005; 41(15): 2268–2279.
5. Krech U. Complement-fixing antibodies against cytomegalovirus in different parts of the world. Bull World Health Organ 1973; 49(1): 103–106.
6. Wreghitt TG, Teare EL, Sule O, Devi R and Rice P. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003; 37(12): 1603–1606.
7. Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, Humar A. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2013; 96(4): 333–360.
8. Brytting M, Xu W, Wahren B and Sundqvist VA. Cytomegalovirus DNA detection in sera from patients with active cytomegalovirus infections. J Clin Microbiol. 1992; 30(8): 1937–1941.
9. Shinkai M, Bozzette SA, Powderly W, Frame P, Spector SA. Utility of urine and leukocyte cultures and plasma DNA polymerase chain reaction for identification of aids patients at risk for developing human cytomegalovirus disease. J Infect Dis. 1997; 175(2): 302–308.
10. Revello MG, Zavattoni M, Sarasini A, Percivalle E, Simoncini L, Gerna G. Human cytomegalovirus in blood of immunocompetent persons during primary infection: Prognostic implications for pregnancy. J Infect Dis. 1998; 177(5): 1170–1175.
11. Chou S. Newer methods for diagnosis of cytomegalovirus infection. Rev Infect Dis 1990; 12 Suppl 7: S727–736.
12. Yoshino T, Nakase H, Ueno S, Uza N, Inoue S, Mikami S, Matsuura M, Ohmori K, Sakurai T, Nagayama S, Hasegawa S, Sakai Y, Chiba T. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm Bowel Dis. 2007; 13(12): 1516–1521.
13. McCoy MH, Post K, Sen JD, Chang HY, Zhao Z, Fan R, Chen S, Leland D, Cheng L, Lin J. qPCR increases sensitivity to detect cytomegalovirus in formalin-fixed, paraffin-embedded tissue of gastrointestinal biopsies. Hum Pathol 2014; 45(1): 48–53.
14. Roblin X, Pillet S, Oussalah A, Berthelot P, Del Tedesco E, Phelip JM, Chambonniere ML, Garraud O, Peyrin-Biroulet L, Pozzetto B. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol. 2011; 106(11): 2001–2008.
The authors
Jingmei Lin* MD PhD and Rong Fan MD PhD
Department of Pathology and Laboratory Medicine, Indiana University School of Medicine, Indianapolis, IN, USA
*Corresponding author
E-mail: jinglin@iupui.edu