The clinical chemistry laboratory in the diagnosis and management of testicular cancer
Cancer of the testicles, primarily the germ cells, is a highly treatable disease common to young men. This article describes how chemical biomarkers are central to the diagnosis, characterization, therapeutic monitoring, prognosis and long-term surveillance in patients with testicular cancer.
by Dr Angela Cooper and Dr Seán Costelloe
Incidence of testicular cancer
Testicular cancer (TC) is relatively rare, accounting for approximately 0.7% of all UK male cancers, with a worldwide incidence estimated as ~7 per 100 000 [1, 2]. Incidence of TC has noticeably increased in industrialized countries over the last few decades, particularly in white males of European descent, although the reasons for this remain unclear [2–5]. Amongst younger men aged between 15 and 49 years in the United Kingdom and the United States of America, TC is the most common type of cancer observed [2, 3, 6, 7].
Classification of TC
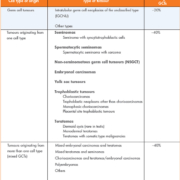
Approximately 95% of malignant TCs originate from primordial germ cells, also known as germ cell tumours (GCTs) [3, 7–9]. However, rarely these malignancies may arise from extragonadal primary sites such as the retroperitoneum, mediastinum or pineal gland [3–5, 8, 10]. Germ cell tumours classified as seminomas (~40%) are predominantly formed of uniform cell types, whereas non-seminomatous germ cell tumours (NSGCTs), also accounting for ~40% of GCTs, originate from multiple cell types such as embryonal carcinomas, teratomas, choriocarcinomas and yolk sac carcinomas. GCTs arising from mixed germ cells comprise the remaining 20%. The World Health Organization (WHO) classification system for testicular tumours (Table 1) define five basic GCT types based on histological examination:
- Seminomatous GCTs
- Non-seminomatous GCTs (NSGCTs)
- Embryonal cell carcinomas
- Yolk sac tumours
- Teratomas
- Choriocarcinoma
The vast majority of non-GCTs are sex cord-gonadal stromal tumours involving the Sertoli or Leydig cells of the testicles, and are often benign [8, 9, 11].
‘Burned-out’ GCTs, or spontaneous regression of a testicular GCT, is a very rare phenomenon occasionally observed in male patients presenting with metastatic malignancy with an absence of primary testicular tumour. Often, the only remaining evidence of malignancy are features such as homogeneous scarring, hemorrhage, intratubular calcification and testicular atrophy. This may be associated with choriocarcinomas or teratomas [5, 12].
Testicular GCTs exhibit very diverse histology and immunostaining profiles, and have varying clinical progression and prognosis outcomes as demonstrated by the numerous methods of GCT classification systems. It is outside the focus of this paper to consider histology or immunostaining used in the identification and differentiation of GCTs, as these topics has been extensively documented in other review articles.
Treatment and cure rates in TC
Advances in treatment strategies, such as the use of cisplatin therapies [13], careful staging at diagnosis, early intervention using multidisciplinary teams, rigorous surveillance follow-up, and salvage therapy, means that GCTs are highly curable. Currently, expected cure rates of 95% are observed in patients who receive a TC diagnosis, and cure rates of 80% in patients with a diagnosis of metastatic TC [3, 13].
Causes and presentation of TC
The causes of TC cancer are still unknown, although cryptochordism is the best-characterized risk factor associated with TC. Research has shown that timing of orchiopexy impacts on future risk of TC development, suggesting hormonal changes during puberty are strongly associated with TC etiology in males. However, prenatal risk factors, environmental exposures in adulthood, male infertility, certain genetic or congenital disorders such as Down’s syndrome, Klinefelter’s syndrome, human immunodeficiency virus infection and intersex patients have also been associated with an increased TC risk [3, 5, 7].
Presentation of TC is often a painless lump in the testis body, but due to a frequent lack of pain, medical opinion is frequently delayed. A testicular mass or swelling, or episodic diffuse pain may be observed. More rarely, metastatic symptoms such back pain arising from retroperitoneal lymph node involvement, or coughing, pain or hemoptysis due to lung metastasis may be reported [3, 7, 8].
Diagnosis and staging of TC
Clinical suspicion of TC, such as altered testicular shape or non-painful swelling, should prompt a full physical examination and patient history, imaging to include testicular and abdominal ultrasound, as well as chest X-ray [14]. If metastasis is suspected, chest, abdominal and brain computerized tomography (CT), and bone scintigraphy should be undertaken [9]. Final diagnosis and prognosis requires biopsy sampling for histology and immunostaining profiling as appropriate, and in the majority of cases, treatment options should be based on the histology results [10]. Biochemical analysis should include initial concentrations of serum tumour markers (STMs). Metabolic biochemistry, liver function tests and a full blood count should be undertaken to determine general organ function, and may demonstrate evidence of metastasis [9].
This collective information can be used to reference the Tumour-node-metastasis (TNM) Classification of Malignant Tumours staging system (Table 2). This cancer staging system is based on primary tumour site, nearby lymph node involvement, and presence of distal metastatic spread from initial primary tumour site [4, 15]. The use of STMs as a fourth staging system has added diagnostic and prognostic value, independent of the TNM system (Table 3) [9]. The decision for chemotherapy or radiotherapy treatment for non-surgical metastatic disease is based on CT and/or magnetic resonance imaging (MRI) results, and concentrations of STMs [4].
The majority of patients (~75%) presenting with a testicular mass are diagnosed at stage 1 [7, 8]. At this stage, treatment options are typically surgery with an excellent cure rate. For metastatic disease, combinations of surgery, chemotherapy or radiotherapy are required depending on cancer mass, location and distal lymph node involvement [13]. Greater than 80% of patients with metastatic GCTs are successfully treated and cured.
Treatment of TC
TC cells are extremely sensitive to chemotherapy [9, 10]. Specifically, the standard chemotherapy regime consists of 3 or 4 cycles of bleomycin, etoposide and cisplatin (BEP) chemotherapy, or etoposide and cisplatin (EP) chemotherapy every 21 days [8, 9]. Surgery may be considered to remove residual masses post-chemotherapy. Data suggests a higher relapse rate in patients with NSGCTs than seminomas following an initial chemotherapy regime. This relapse rate can be used to further classify patients into good, intermediate and poor prognostic groups, using a combination of STM concentrations and location of primary tumour or metastases. Around 50–99% of patients can still expect to survive [8].
Salvage therapy, often in combination with chemotherapy, is reserved for patients who have relapsed, or for patients where cancer progression continues after following a standard chemotherapy regime. High-dose chemotherapy with autologous bone marrow transplant is a controversial approach for patients with a poor prognosis, and where a standard chemotherapy regime and salvage therapy has been unsuccessful. Initial studies are encouraging but further trials are required. A small cohort of patients have been identified who suffer a late relapse, i.e. >2 years post-diagnosis but also potentially ≥10 years post-diagnosis. These patients are less responsive to chemotherapy, so are treated primarily with surgery. Unfortunately, less than half will remain disease-free following surgical intervention [8, 9]. Chemotherapy-induced side effects are governed by the dose and combination of drugs used. This has triggered more recent trials designed at maintaining a cure rate but with reduced associated chemotoxicity [8].
The use of serum tumour markers in TC
The discovery of serum and urine tumour markers and the advent of chemotherapy have significantly improved cancer staging, management and prognosis in patients with TC. The benefit of initial STMs is predominantly with regard to disease staging, whereas serial STMs are particularly useful for monitoring response to treatment after surgery, chemotherapy or radiation therapy. STMs are useful because they are often detectable well before clinical radiological detection in patients. Furthermore, concentrations can be helpful to differentiate GCT type. The detection of at least one elevated STM occurs in ~85% of NSGCTs, and the presence of elevated STMs occurs in significant numbers of pure seminoma cases [9, 10]. However, in rare cases where patients present with evidence of a testicular mass, radiographic evidence of metastatic disease, with significantly elevated alpha-fetoprotein (AFP) or human chorionic gonadotrophin (hCG) serum concentrations, it is advised that treatment is not delayed while awaiting histology results [10].
The American Society of Clinical Oncology recommend against using STMs as a screening test for GCTs in asymptomatic males. Given the low incidence and mortality of TC combined with the high cure rate, it is suggested a screening programme would be neither cost-effective nor decrease mortality [10]. Furthermore, although STMs can be helpful in combination with imaging techniques in the diagnosis of TC, normal STMs alone do not exclude TC and may also be raised in other conditions [3, 8–10]. Routine testicular examination via palpation is recommended in all males from puberty up to ~45 years. This is of particular importance for males with a past medical history that may suggest an increased GCT risk as detailed previously.
Commonly employed serum markers include: AFP and hCG as mentioned previously, hCG beta-subunit (hCGb), placental alkaline phosphatase (PLAP) and lactate dehydrogenase (LDH). Alpha-fetoprotein levels are elevated in teratocarcinoma or testicular embryonal carcinoma, while conversely, AFP is never elevated in pure seminomas. Human chorionic gonadotrophin elevations are associated with 10–15 % of pure seminomas. Lactate dehydrogenase is an enzyme found in all cell types, meaning it is less specific for TC, although it does have prognostic value in advanced stage GCTs [3, 9]. A decline in serial STM concentrations is useful to detect the presence of residual disease following surgery, or to assess response to chemotherapy. In both scenarios, the decline in STM concentrations should follow the half-lives of each marker [9].
There are detailed STM surveillance guidelines in place following surgery, which recommend a meticulous timetable of STM measurements and radiology imaging to detect disease recurrence depending on initial GCT type, thereby avoiding relapse and presentation at a later date with advanced stage disease [8, 9].
Future focus
While the majority of patients diagnosed with TC will survive, challenges still persist. Serum tumours markers have been pivotal to improved outcomes for patients with and without metastatic disease. Future research is focused on patients with an initial poorer prognosis, patients who have relapsed following first-line chemotherapy and patients who have a late relapse. Long-term health consequences for patients surviving TC, in particular side effects associated with chemotherapy and radiotherapy such as cardiovascular disease, impaired fertility and secondary cancers, continues to drive collaborative studies nationally and internationally to improve TC outcomes for the future.
References
1. Cancer registration statistics, first release, England, 2014. Office for National Statistics 2014. (http://web.ons.gov.uk/ons/rel/vsob1/cancer-statistics-registrations–england–series-mb1-/2014–first-release-/rpt-cancer-stats-registrations.html)
2. Hameed A, White B, Chinegwundoh F, Thwaini A, Pahuja A. A review in management of testicular cancer: single centre review. World J Oncol. 2011; 2: 94–101.
3. Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997; 337: 242–254.
4. Bahrami A, Ro JY, Ayala AG. An overview of testicular germ cell tumors. Arch Pathol Lab Med. 2007; 131: 1267–1280.
5. Sesterhenn IA,Davis, CJ. Pathology of germ cell tumors of the testis. Cancer Control 2004; 11: 374–387.
6. Wu X, Groves FD, McLaughlin CC, Jemal A, Martin J, Chen, VW. Cancer incidence patterns among adolescents and young adults in the United States. Cancer Causes Control. 2005; 3: 309–320.
7. Hanna NH, Einhorn LH. Testicular cancer – discoveries and updates. N Engl J Med. 2014; 371: 2005–2016.
8. Horwich A, Nicol D,Huddart R. Testicular germ cell tumours. BMJ 2013; 347: f5526.
9. Barlow LJ, Badalato GM,McKiernan JM. Serum tumor markers in the evaluation of male germ cell tumours. Nat Rev Urol. 2010; 7: 610–617.
10. Gilligan TD, Hayes DF, Seidenfeld J, Temin S. ASCO clinical practice guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010; 6: 199–202.
11. Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World Health Organization classification of tumours. Pathology and genetics of tumours of the urinary system and male genital organs. IARC 2004.
12. Ulbright TM. Germ cell tumours of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Mod Pathol. 2005; 18: S61–S79.
13. Masters JR, Köberle B. Curing metastatic cancer: lessons from testicular germ-cell tumours. Nat Rev Cancer. 2003; 3:517–525.
14. Suspected cancer: recognition and referral guidelines [NG12]. National Institute for Health and Care Excellence (NICE) 2015. (https://www.nice.org.uk/guidance/NG12/chapter/1-Recommendations-organised-by-site-of-cancer)
15. Sobin LH, Gospodarowicz MK and Wittekind C. TNM classification of malignant tumours (7th ed). International Union against Cancer (UICC). Wiley-Blackwell 2009.
16. Albers P. (Chair), Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, Horwich A, Laguna MP, Nicolai N, Oldenburg J. Guidelines on testicular cancer. Eur Urol. 2015. (https://uroweb.org/guideline/testicular-cancer/)
The authors
Angela Cooper* PhD, Seán Costelloe, PhD
Derriford Combined Laboratory, Plymouth Hospital NHS Trust, Plymouth, UK
*Corresponding author
E-mail: angelacooper5@nhs.net