Therapeutic drug monitoring of methadone
Methadone maintenance therapy is central to the treatment of opiate dependence. Assessment of adherence is essential to ensure success and to prevent misuse of prescribed medications. A variety of specimen types can be tested for methadone and its main metabolite using a number of different analytical methods. The benefits and limitation associated with each are discussed.
by Dr Elizabeth Fox and Dr Deepak Chandrajay
Introduction
Opiate dependence is an important problem worldwide. In the UK, individuals seeking help with their addiction are referred to substance misuse services where they are usually offered methadone or buprenorphine substitution therapy [1]. Methadone is a synthetic opioid with pharmacological actions similar to opiates mediated through the mu receptor. Treatment is initiated at a dose of 10–40 mg daily and gradually increased by 10–20 mg weekly. The usual maintenance dose is 60–120 mg daily, but some clients require a higher dose for symptomatic relief [2]. Its long half-life allows for a once-daily dosing schedule and the accumulation in the body means that steady-state plasma concentrations are easily achieved after repeated administration.
Methadone reduces or eliminates withdrawal symptoms and helps the subject reach a drug-free state in a controlled way. There is evidence of reduced illicit opiate misuse, criminal activity and mortality when patients are on maintenance therapy [2, 3]. Injecting behaviours and incidence of HIV infection are also reduced [4].
Methadone is a lipid soluble drug with an oral bioavailability of approximately 95%. It is metabolized by cytochrome P-450 (CYP) enzymes and demethylated to 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP). Both parent drug and metabolite are excreted in the urine and can also be detected in blood, oral fluid, sweat and hair. Co-administration of CYP enzyme inducing drugs such as rifampicin, phenytoin, and zidovudine can precipitate opiate withdrawal symptoms. Fluoxetine and fluvoxamine can inhibit CYP enzymes and have an opposite effect on methadone metabolism [5].
In contrast to most other forms of therapeutic drug monitoring where blood concentration is maintained within a narrow therapeutic window, methadone is monitored almost exclusively to confirm adherence with the treatment regimen. The client may seek to falsify the drug test to feign adherence when the drug is actually being sold to others, or simply to mask illicit drug use. Such individuals will submit a specimen spiked with methadone mixture, therefore effective methods for methadone testing should use matrices which are resistant to tampering and/or include measures to detect falsified samples. Absence of EDDP from a methadone-positive urine sample strongly suggests that it has been spiked with medication. Measurement of urine creatinine will identify samples which have been diluted or substituted, for example with tea. Methadone mixture is green so a green tinge to urine should raise suspicions of sample spiking. The temperature and pH of fresh urine specimens can be recorded to assess reliability. Salivary IgG is useful to confirm integrity of oral fluid specimens.
Analytical methods used for methadone testing
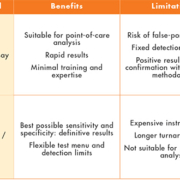
The key analytical methods used to measure methadone and EDDP are immunoassay, liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) and gas chromatography coupled to mass spectrometry (GC-MS). The benefits and limitations of each are summarized in Table 1. Immunoassay is rapid, high throughput when automated and low cost. Sensitivity is determined by the detection cut-off concentration of the test kit and specificity by the specific antibody used in the kit. Point-of-care test (POCT) kits are available for use with urine and oral fluid in the clinic and require little expertise or training. POCT offers the significant advantage of producing instant results that can be discussed during the consultation. Laboratory-based immunoassays can be run on multichannel clinical chemistry analysers and do not require additional staff training. All immunoassay-based techniques are prone to interference from unrelated compounds due to cross-reaction with the specific antibody. Cross reactivity data are available from the manufacturer and should be borne in mind when interpreting results. False-positive methadone results have been documented with diphenhydramine, doxylamine and phenothiazines [6]. Immunoassay-based tests, whether designed for POCT or laboratory use, are sold as screening tests. The manufacturers recommend the confirmation of positive results using an alternative methodology such as LC-MS/MS or GC-MS. That said, not all laboratories and substance misuse clinics routinely confirm methadone and EDDP positive results. UK Department of Health guidance on adherence testing recommends only that positive screen tests are confirmed ‘if appropriate’[1].
Mass spectrometric techniques offer the best possible sensitivity and specificity and are considered the ‘gold standard’. Test menus are user-defined which allows simultaneous detection of methadone and EDDP and any other drug as required. Disadvantages of these techniques are that they require expensive specialist instrumentation, labour intensive sample preparation, and complex data interpretation. Turnaround times can be lengthy and they are not amenable to POCT. LC-MS/MS methods require considerably less sample preparation than GC-MS and are now used in many clinical laboratories. A few labs including our own have adopted LC-MS/MS for first-line drug screening of urine and oral fluid specimens.
Specimen types for methadone testing
The main sample types are summarized in Table 2.
Urine
Regular urine testing is the most commonly used means of confirming adherence with methadone prescription. The advantages of urine are that both methadone and EDDP are readily detected and collection is easy and non-invasive. Presence of EDDP provides proof that methadone has been ingested and not spiked into the sample. A disadvantage of urine is that it is easy to manipulate. A sample of donor urine, if the donor is taking methadone, submitted in place of the patient’s own is difficult to recognize. Supervised collection is not always desirable as it is an invasion of privacy and subjects may suffer from ‘shy bladder’. The concentrations of methadone and EDDP do not correlate well with dose (because of the variability of untimed urine samples), so qualitative urine analysis is only suitable for confirming use.
Commercially available methadone and EDDP immunoassays typically have a fixed cut-off or detection threshold of between 100 and 300 µg/L. Multidrug panel tests usually include either methadone or EDDP. Choosing a test which specifically detects EDDP will minimize the chance that a spiked urine is passed off as positive. An estimated 4% of methadone-positive samples submitted to our laboratory lack detectable EDDP; a methadone-only assay would not identify these specimens (unpublished observation). Our approach to urine testing is to measure both methadone and EDDP by LC-MS/MS in all samples. The testing strategy in an increasing number of substance misuse clinics is to use POCT as a first-line test, then to refer suspicious or disputed samples to the lab for confirmation. A laboratory immunoassay would offer no further information for samples that have already been tested at the point of care and could theoretically suffer the same interference.
Oral fluid
An alternative matrix for methadone testing is oral fluid. The advantage of oral fluid is that collection is simple, easily observable and can be done in the consultation room. POCT devices are available for instant results or samples can be sent for laboratory analysis. Both immunoassay and mass spectrometric analytical methods are available. The amount of methadone and EDDP present in oral fluid is dependent upon salivary pH. Methadone is a basic drug and under acidic conditions it becomes ionized and ‘trapped’ in the saliva. Unstimulated saliva is more acidic than stimulated saliva so false negatives can be avoided by asking the subject to abstain from eating, drinking or chewing for 10 minutes prior to collection. A recent study involving subjects on daily methadone doses found that the concentration of methadone in saliva correlated poorly with dose and that EDDP was below detection in 12% of samples [7]. However, methadone was readily detectable in all samples suggesting that oral fluid is a useful specimen for confirming adherence. Oral fluid methadone does not reflect the plasma concentration so would not be useful for assessing dose adequacy. Contamination with methadone from the oral cavity is a problem and absence of EDDP, if measured, should be interpreted with caution as it does not necessarily equate to sample adulteration.
Blood
The main advantage of using blood to monitor methadone therapy is that it’s virtually impossible to falsify the sample. Plasma concentration correlates with methadone dose but the concentrations at which therapeutic effect is achieved have not been well defined. Several studies have suggested target concentrations; other studies have found no correlation between plasma concentration and either heroin use or opiate withdrawal symptoms [8]. A further study suggested that the pharmacodynamics of methadone can be altered by the presence of other drugs therefore altering the relationship between plasma methadone and effect [9]. There is debate in the literature as to whether plasma concentration is any more useful than daily dose for predicting response to treatment [10]. Given the polypharmacy present in the majority of subjects receiving methadone, routine use of plasma methadone to titrate dose is likely to need further evaluation. Intravenous drug users tend to have poor venous access so collecting samples may be challenging. Methods using dried blood-spot samples to circumvent this problem have been described but skin contamination with methadone is likely to be an issue [11]. Blood is not the ideal specimen to assess use of other substances because of the very short detection window, so additional testing may be required. In conclusion, blood testing is best reserved for difficult cases where knowledge of the plasma concentration may be helpful.
Other matrices
Monitoring of methadone therapy using sweat analysis has been evaluated. Patches are typically worn for up to 7 days then dispatched to the laboratory for analysis. They are tamper-evident and claim to be difficult to adulterate. Large inter- and intra-individual variations in sweat methadone concentration have been observed and there is only a weak correlation between patch concentration and dose. Sweat testing is, however, useful for detecting exposure to other substances so may be applicable to some cases. Hair analysis can be used to retrospectively confirm adherence with methadone treatment but is not useful for real-time assessment.
Concluding remarks
The current trend is for substance misuse services to perform methadone adherence testing in the clinic and refer samples to the laboratory for confirmation where necessary. Substance misuse clinic personnel are not laboratory scientists, therefore a key role of the laboratory that performs confirmatory testing is to develop a good working relationship and ensure all aspects of testing are fully understood.
References
1. Department of Health (England) and the devolved administrations. Drug Misuse and Dependence: UK Guidelines on Clinical Management. London: Department of Health (England), the Scottish Government, Welsh Assembly Government and Northern Ireland Executive. 2007; www.nta.nhs.uk/uploads/clinical_guidelines_2007.pdf.
2. National Institute for Health and Clinical Excellence. Methadone and buprenorphine for the management of opioid dependence. Technology appraisal guidance 114. NICE 2007; http://guidance.nice.org.uk/TA114.
3. Advisory Council on the Misuse of Drugs. Reducing drug-related deaths: a report by the Advisory Council on the Misuse of Drugs. ACMD, Home Office 2000; ISBN 0-11-341239-8.
4. NTORS, The National Treatment Outcome Research study. 2001; http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_4084908.
5. McCance-Katz EF, Sullivan L and Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine and other frequently prescribed medications: a review. Am J Addict. 2010; 19(1): 4–16.
6. Lancelin F, Kraoul L, Flatischler N, Brovedani-Rousset S, Piketty ML. False-positive results in the detection of methadone in urines of patients treated with psychotropic substances. Clin Chem. 2005; 51(11): 2176–2177.
7. Gray TR, Dams R, Choo RE, Jones HE, Heustis MA. Methadone disposition in oral fluid during pharmacotherapy for opioid-dependence. Forensic Sci Int. 2011; 206 (1–3): 98–102.
8. Shiu JR, Ensom MHH. Dosing and monitoring of methadone in pregnancy: literature review. Can J Hosp Pharm. 2012; 65(5): 380–386.
9. Kharasch ED, Walker A, Whittington D, Hoffer C, Sheffels Beynek P. Methadone metabolism and clearance are induced by nelfinavir despite inhibition of cytochrome P4503A (CYP3A) activity. Drug Alcohol Depend. 2009; 101(3): 158–168.
10. Hallinan R, Ray J, Byrne A, Agho K, Attia J. Therapeutic thresholds in methadone maintenance treatment: a receiver operating characteristic analysis. Drug Alcohol Depend. 2006; 81(2): 129–136.
11. Saracino MA, Marcheselli C, Somaini L, Pieri MC, Gerra G, et al. A novel test using dried blood spots for the chromatographic assay of methadone. Anal Bioanal Chem. 2012; 404(2): 503–511.
The authors
Liz Fox* PhD, FRCPath and Deepak Chandrajay MBBS, MRCP
Specialist Laboratory Medicine, St James’s University Hospital, Leeds, UK
*Corresponding author
E-mail: Elizabeth.fox@leedsth.nhs.uk