Touchlight launches circular DNA architectures for gene therapy
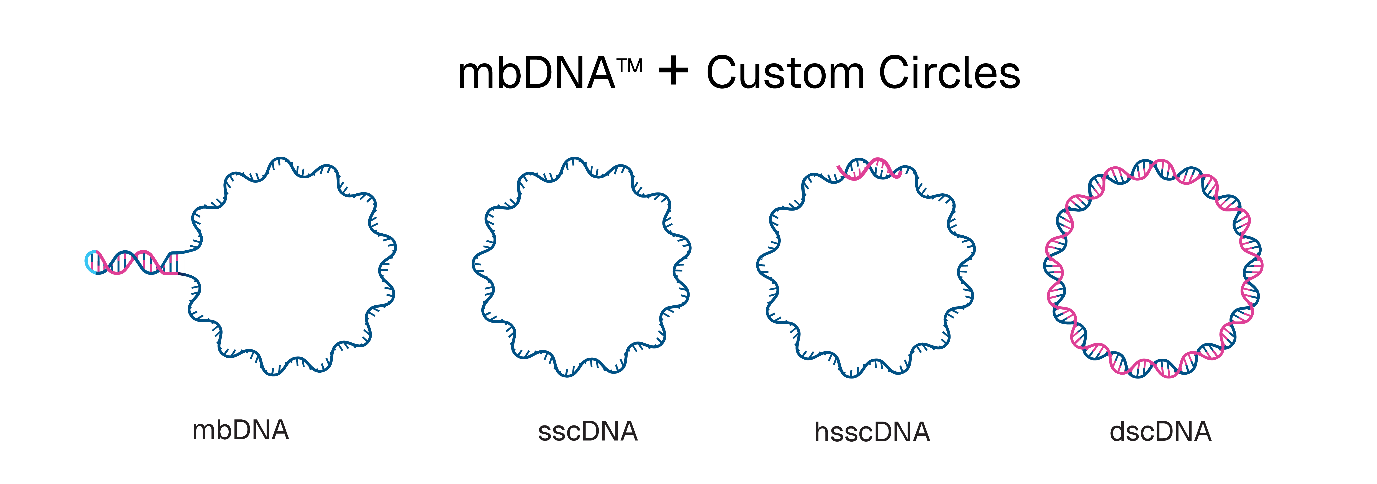
Touchlight has expanded its mbDNA (megabulb DNA) platform with three new circular DNA architectures designed to enhance gene editing and therapeutic development. The company says that sscDNA, hsscDNA, and dscDNA constructs now complement its existing mbDNA technology, offering researchers customisable solutions for gene insertion and expression applications.
Cell-free enzymatic production platform
The mbDNA platform utilises Touchlight’s cell-free enzymatic production system to generate single-stranded DNA with minimal immunogenicity. According to the company, mbDNA achieves knock-in rates of 60-75% in primary T cells through homology-directed repair (HDR) whilst maintaining low toxicity levels. The technology supports payload capacities up to 20 kb, enabling delivery of large genetic sequences for gene editing applications.
Customisable DNA architectures for diverse applications
The newly introduced architectures provide flexibility for different therapeutic platforms. sscDNA is a fully single-stranded circular DNA molecule, whilst hsscDNA contains bespoke double-stranded regions of user-defined length. dscDNA represents a fully double-stranded circular molecule. All three constructs feature completely user-defined sequences suitable for transposases, recombinases, homology-independent targeted integration (HITI), and episomal expression.
“mbDNA is a market leading technology providing the best-in-class HDR template available today,” said Karen Fallen, CEO at Touchlight. “With the addition of sscDNA, hsscDNA, and dscDNA, we’re empowering researchers and developers to unlock new possibilities in gene editing and therapeutic innovation.”
Manufacturing capabilities and regulatory status
Touchlight operates as a contract development and manufacturing organisation (CDMO) specialising in enzymatic GMP DNA production. The company received FDA Drug Master File acceptance in 2022 and was awarded the world’s first cell-free DNA GMP licence in 2025. Multiple client products utilising Touchlight’s DNA manufacturing technology are currently in clinical development.
The expanded portfolio addresses limitations of traditional plasmid DNA production methods, which rely on bacterial fermentation. Touchlight’s enzymatic approach offers an alternative manufacturing process for viral vectors, mRNA, non-viral gene therapy, DNA vaccines, and gene editing applications across pre-clinical, clinical, and commercial stages.
Founded in 2007, Touchlight is headquartered in London and focuses on synthetic DNA manufacturing solutions for genetic medicine development.
For more information, visit: https://www.touchlight.com
Digital issue: Please click here for more information