Trimero’s κλoneus kits offer alternative approach for Bence-Jones proteinuria detection
κλoneus Free Light Chain (FLC) kits from Spanish in vitro diagnostics manufacturer Trimero Diagnostics SL offer clinical laboratories an alternative approach for determining Bence-Jones proteinuria based on International Federation of Clinical Chemistry (IFCC) guidelines.
The κλoneus assays use nephelometric or turbidimetric methods for quantifying free light chains in serum and urine samples, compatible with major analysers currently used for this type of protein analysis.
Dual utility for urine analysis
The measurement of FLCs in urine serves two distinct purposes, according to the company. Qualitatively, κλoneus functions as a precise and highly sensitive method for indicating the presence of FLCs in samples, with any possible monoclonal nature requiring confirmation through electrophoretic methods such as immunofixation or immunosubtraction. Quantitatively, the assay provides an estimate of Bence-Jones proteinuria concentration, enabling patient follow-up. The company says that κλoneus FLC results correlate with densitometric estimation of Bence-Jones proteinuria whilst being more sensitive and precise, facilitating improved monitoring.
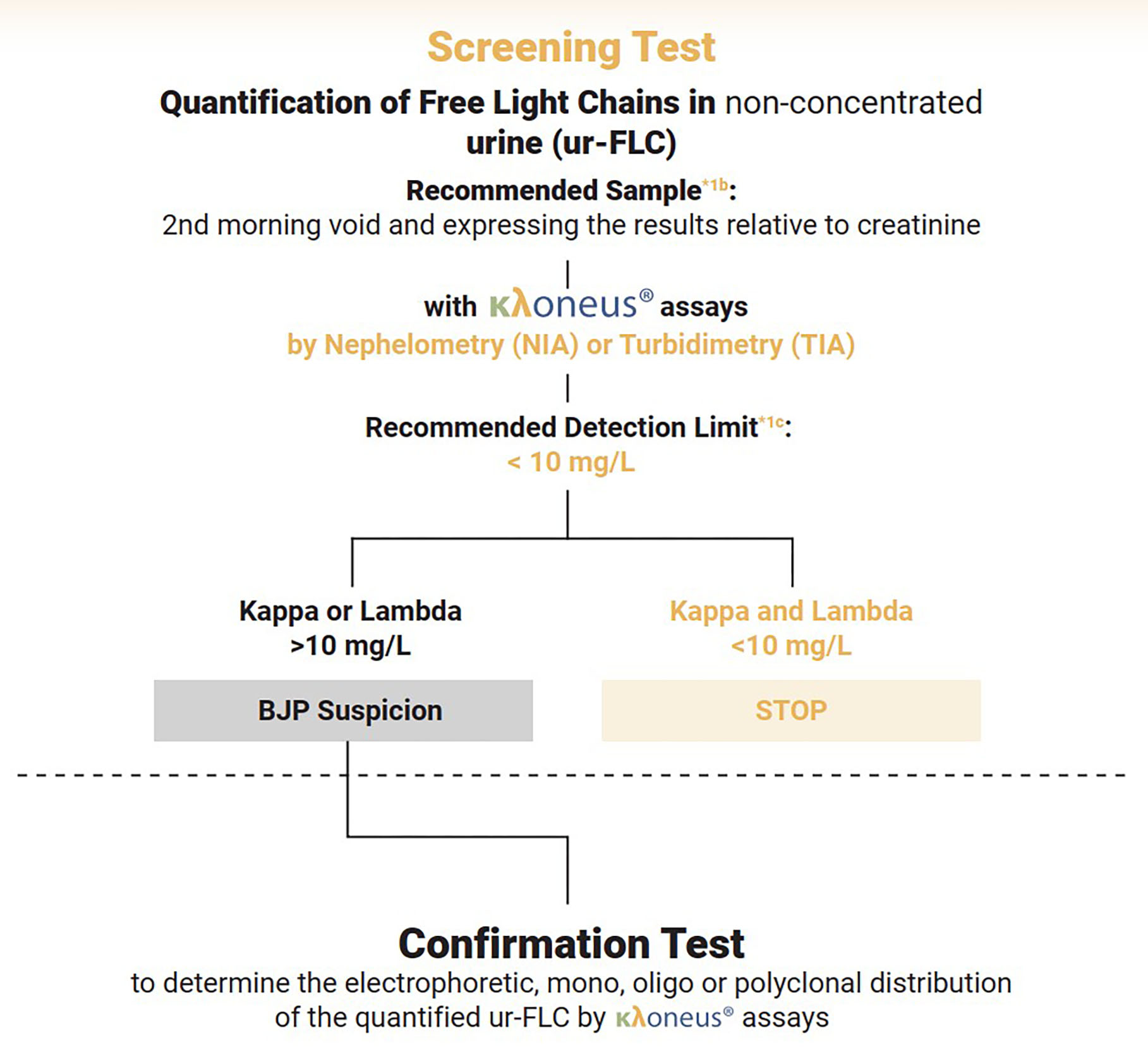
Addressing clinical guidelines
Most guidelines for monoclonal gammopathies maintain that urine analysis remains necessary. The IFCC-based screening protocol involves quantifying free light chains using the κλoneus assay, with testing discontinued if both kappa and lambda measurements fall below cut-off values. Cases exceeding these thresholds proceed to confirmatory electrophoretic assays.
For serum applications, Trimero notes that both the performance and limitations of its κλoneus kits are comparable to equivalent products available on the market.
Company background and partnerships
Trimero Diagnostics SL specialises in producing IVD devices for determining specific proteins not typically available in major manufacturers’ catalogues. The company has obtained In Vitro Diagnostic Regulation (IVDR) certification and is managed by a team with more than 35 years of experience in the protein sector.
Major diagnostic companies including Siemens Healthineers, Binding Site (part of Thermo Fisher), and Beckman Coulter market selected Trimero Diagnostics products to complement their commercial offerings.
For more information, visit: www.kloneus.com
Digital issue: Please click here for more information