Using HCV core antigen testing to improve diagnosis of acute infection
Many people become infected with hepatitis C virus (HCV) every year and these infections often have no symptoms. A significant number of patients will go on to develop chronic liver disease and potentially hepatocellular carcinoma. Early detection of HCV infection is of great importance, but remains challenging. This article describes the advantages and limitations of methods of HCV diagnosis.
by Dr O. Blach, Dr D. Lawrence, Dr F. Cresswell and Prof. M. Fisher
Hepatitis C virus infection
It is estimated that 3% of the world’s population has hepatitis C virus (HCV), with a further 3–4 million people becoming newly infected every year [1]. Early detection of HCV infection is of great importance, as prompt diagnosis enables contact tracing, partner notification, health promotion advice to reduce the risk of onward transmission and disease progression, and the opportunity for early treatment, which may offer the best opportunity for cure [2].
However, diagnosing acute hepatitis C remains challenging. Most patients with acute infections are asymptomatic, and even when symptoms are present, they are often non-specific, not severe, and may not present in the same way as those with other hepatitis viruses (such as A, B and E). Approximately 10–20% of patients clear the virus spontaneously during acute infection; the remainder progress to chronic infection which, if unrecognized, will progress in a significant proportion to chronic liver disease, cirrhosis, end-stage liver disease and hepatocellular carcinoma [2]. Although newer directly acting antiviral drugs (DAAs) against HCV will transform management, for many individuals pegylated interferon and ribavirin may remain standard of care for some time until these can be afforded. Therefore early diagnosis for many will offer the best opportunity for cure at the present time.
Established diagnostic tests
The diagnosis of acute hepatitis C is usually made after detection of abnormal liver function tests or on routine screening in specific populations, such as those with HIV infection, on hemodialysis for end-stage renal failure, or accessing services for injecting drug users. Traditionally, seroconversion from anti-HCV antibody (anti-HCV) negative to positive, a process which takes places around 12 weeks after infection, is detected by enzyme-linked immunosorbent assays (ELISA, EIA) or chemiluminescence immunoassay (CIA) [3].
However, although the presence of anti-HCV indicates infection with HCV at some point, it does not determine whether it is acute, chronic or resolved. Furthermore, anti-HCV may not be detectable during this aforementioned 12-week ‘window period’, or if the patient is immunocompromised and therefore has an impaired ability to produce antibodies [4], with delayed seroconversion up to 18 months being reported [5].
Detection of viremia in the setting of a negative anti-HCV (during the seronegative ‘window period’), and therefore verification of active HCV infection has historically been done using nucleic acid amplification test (NAAT) for HCV RNA by quantitative reverse transcription polymerase chain reaction (qRT-PCR), which can detect HCV RNA in serum 1–3 weeks after infection [6–8]. Although the ‘gold standard’ for diagnosing acute HCV infection, HCV qRT-PCR has several shortcomings: it is costly, labour-intensive, time-consuming and requires advanced technical skills, separate facilities (separate platform) and equipment [9], which make it particularly impractical in a resource-poor setting. As a consequence, HCV core antigen (Ag) quantification as a surrogate marker of HCV replication has been suggested as an alternative assay for initial testing of acute hepatitis [10].
HCV core antigen
HCV core Ag is part of the HCV capsid formed by core protein polymerization, and as such, is one of the best ‘conserved’ products of viral genome [11]. Using HCV core Ag, acute infection with HCV can be detected in the serum earlier than with the current anti-HCV screening assays [12], and only 1–2 days later than with HCV RNA NAAT tests [13].
Since the development of the first HCV core Ag tests around 2000, newer assays, which are up to 25 times more sensitive, have become available and are licensed in several countries. A recent meta-analysis of 25 studies conducted by Gu et al. [14] compared the diagnostic accuracy of HCV core Ag (index reference) with HCV RNA (‘gold standard’) and showed good pooled sensitivity of 0.84 (95% CI, 0.83–0.85), with excellent pooled specificity of 0.98 (95% CI, 0.97–0.98) for HCV core Ag assays. HCV core Ag can therefore be used as a marker of viraemia [7] with the lower limit of detection corresponding to HCV RNA load of 700–1100 IU/mL [15]. Positive and negative predictive values reported in the literature for HCV core Ag assays are also high, with one study reporting PPV of 100% and NPV of 97% [16]. However, re-testing samples with low positive Ag values (<35fM) has been recommended after one study by Shepherd et al. [17] reported 37% false positive rates with such results. Another study by Cresswell et al. [7] recorded two false-indeterminate results, one of which was false positive on re-testing.
Furthermore, HCV core Ag levels closely track those of HCV RNA with multiple studies identifying a strong non-linear correlation between the two, thus potentially also allowing clinical monitoring of a patient’s therapy, independently of HCV genotype [15]. This is mainly the case in samples with HCV RNA levels above 20 000 IU/ml, thereby limiting their use in practice [18].
Given its slightly lower sensitivity compared with HCV RNA PCR, the utility of HCV core Ag testing in a diagnostic algorithm for acute hepatitis C is dependent on the practicalities of testing in a given population setting and the potential cost savings [19]. One appealing advantage of HCV core Ag assays lies in the potential for reflex HCV core Ag testing in anti-HCV positive samples using the same testing platform and the same sample [20], thus providing physicians with clinically meaningful results of both anti-HCV and HCV core Ag within an hour.
HCV core Ag could also prove to be more stable than HCV RNA in situations where testing cannot be done on a fresh sample or where a sample needs to be transported to another laboratory [21]. As such, HCV antigen detection could be a viable next step following a positive anti-HCV test, and additional HCV RNA testing would only be necessary with negative or low positive HCV core Ag values.
Furthermore, besides a faster processing time compared to traditional molecular tests, HCV core Ag assays are cheaper [22] and thereby especially attractive in low-resource settings or where PCR may be unavailable [9]. Cresswell et al. estimated potential cost savings of $18 275 in equipment and $6964 in manpower per year in an HIV cohort of 2200 people, had HCV core Ag been used in place of HCV RNA PCR [7].
Special populations
The usefulness of HCV core Ag as a screening investigation in the immunocompromised cohort has attracted considerable attention recently, given their impaired antibody production and the well-recognized delay in HCV antibody seroconversion [5]. High sensitivities (100%) and specificities (97.9 and 97.7%) were reported by Cresswell et al. [7] and Carney et al. [23] in diagnosing acute hepatitis C in HIV-infected individuals by HCV core Ag. Another study of dialysis patients by Moini et al. found only one HCV RNA positive patient to be HCV core Ag negative (note a low HCV viral load of <100 IU/mL) [24].
Finally, in the context of blood transfusion or organ transplantation, the modern HCV RNA assays remain the most sensitive and preferred option [25], but in a resource-limited blood bank setting, testing with HCV core Ag might be superior to no testing for HCV viremia at all. Further research is needed to determine the role of HCV core Ag testing in monitoring of both the untreated patients and those undergoing therapy, as well as in predicting the histological chances and disease progression.
Looking to the future
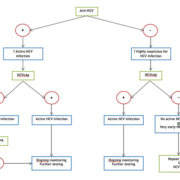
In conclusion, given the inadequacies of HCV antibody testing in acute infection and the time and financial constraints of HCV RNA PCR, HCV core Ag detection offers a new, cheaper and effective way of testing for acute hepatitis C, and is a promising confirmatory test for anti-HCV positive patients. Given the emerging evidence on the constantly improving HCV core Ag assays, we believe that national guidelines should now begin to consider HCV core Ag testing as an integral part of the HCV screening algorithm for acute HCV infection, as illustrated in Figure 1.
References
1. World Health Organization. Secretariat. Viral hepatitis. Sixty-Third World Health Assembly A63/15. Provisional agenda item 11.12. 25 March 2010. World Health Organization, 2010.
2. Webster DP, Klenerman P, Collier J, Jeffery KJ. Development of novel treatments for hepatitis C. Lancet Infect Dis. 2009; 9(2): 108–117.
3. Pondé RA. Enzyme-linked immunosorbent/chemiluminescence assays, recombinant immunoblot assays and nucleic acid tests in the diagnosis of HCV infection. Eur J Clin Microbiol Infect Dis. 2013; 32(8): 985–988.
4. Chamot E, Hirschel B, Wintsch J, et al. Loss of antibodies against hepatitis C virus in HIV-seropositive intravenous drug users. AIDS 1990; 4(12): 1275–1277.
5. Thomson EC, Nastouli E, Main J, et al. Delayed anti-HCV antibody response in HIV-positive men acutely infected with HCV. AIDS 2009; 23: 89–93.
6. Cox AL, Netski DM, Mosbruger T, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Inf Dis. 2005; 40: 951–958.
7. Cresswell F, Fisher M, Hughes D, et al. Hepatitis C core antigen testing: a reliable, quick, and potentially cost-effective alternative to hepatitis c polymerase chain reaction in diagnosing acute hepatitis C virus infection. Clin Inf Dis. 2015; 60(2): 263–266.
8. Umar M, Khan A, Abbas Z, et al. World Gastroenterology Organisation global guidelines: diagnosis, management and prevention of hepatitis C April 2013. J Clin Gastroenterol. 2014; 48(3): 204–217.
9. Chakravarti A, Chauhan MS, Dogra G, et al. Hepatitis C virus core antigen assay: can we think beyond convention in resource limited settings? Braz J Infect Dis. 2013; 17(3): 369–374.
10. Hadziyannis E, Minopetrou M, Georgiou A, et al. Is HCV core antigen a reliable marker of viral load? An evaluation of HCV core antigen automated immunoassay. Ann Gastroenterol. 2013; 26(2): 146–149.
11. Caruntu F, Benea L. Acute Hepatitis C Virus Infection: Diagnosis, Pathogenesis, Treatment. J Gastrointestin Liver Dis. 2006; 15(3): 249–256.
12. Dawson G. The potential role of HCV core antigen testing in diagnosing HCV infection. Antivir Ther. 2012; 17: 1431–1435.
13. Heathcote J, et al. World Gastroenterology Organisation Practice Guidelines: Management of acute viral hepatitis. (December 2003). http://www.worldgastroenterology.org/assets/downloads/en/pdf/guidelines/02_acute_hepatitis.pdf visited on 22nd Jan 2015.
14. Gu S, Liu J, Zhang H, et al. Core antigen tests for hepatitis C virus: a meta-analysis. Mol Biol Rep. 2012; 39: 8197–8208.
15. Medici MC, Furlini G, Rodella A, et al. Hepatitis C virus core antigen: analytical performances, correlation with viraemia and potential applications of a quantitative, automated immunoassay. J Clin Virol. 2011; 51: 264–269.
16. Li Cavoli G, Zagarrigo C, Schillaci O, et al. Hepatitis C Virus core antigen test in monitoring of dialysis patients. Hepat Res Treat. 2012; 2012: 832021.
17. Shepherd S, Aitken C, Walkowicz M, et al. HCV antigen testing in a busy diagnostic laboratory. Clinical Microbiology and Infection 2012; 18: 676–77.
18. Pawlotski JM. Use and interpretation of virological tests for hepatitis C. Hepatology 2002; 36(5,S1): S65–S73.
19. Tillmann H. Hepatitis C virus core antigen testing: role in diagnosis, disease monitoring and treatment. World J Gastroenterol. 2014; 20(22): 6701–6706.
20. Ottiger C, Gygli N, Huber AR. Detection limit of architect hepatitis C core antigen assay in correlation with HCV RNA, and renewed confirmation algorithm for reactive anti-HCV samples. J Clin Virol. 2013; 58: 535–540.
21. Miedouge M, Saune K, Kamar N, et al. Analytical evaluation of HCV core antigen and interest for HCV screening in haemodialysis patients. J Clin Virol. 2010; 48: 18–21.
22. Tedder RS, Tuke P, Wallis N, et al. Therapy-induced clearance of HCV core antigen from plasma predicts an end of treatment viral response. J Viral Hepat. 2013; 20: 65–71.
23. Carney R, Maranao D, Sudra R, et al. A hepatitis C virus core antigen assay is a cost-effective, sensitive and specific test in the detection of acute hepatitis C in HIV infected subjects. HIV Med. 2014; 15(S3): 8.
24. Moini M, Ziyaeyan M, Aghaei S, et al. Hepatitis C virus (HCV) infection rate among seronegative hemodialysis patients screened by two methods; HCV core antigen and polymerase chain reaction. Hepat Mon. 2013; 13: e9147.
25. Waldenström J, Konar J, Ekermo B, et al. Neonatal transfusion-transmitted hepatitis C virus infection following a pre-seroconversion window-phase donation in Sweden. Scand J Infect Dis. 2013; 45: 796–99.
The authors
Ola Blach*1 MBChB, BSc; David Lawrence1 MBChB, MSc; Fiona Cresswell1 MD, MBBS; and Martin Fisher1,2 FRCP, MBBS, BSc
1Lawson Unit, Department of HIV and Sexual Health, Royal Sussex County Hospital, Brighton, UK
2Brighton and Sussex Medical School, Brighton, UK
*Corresponding author
E-mail: ola.blach@doctors.org.uk