24,25-dihydroxyvitamin D: a new biomarker in non-parathyroid hypercalcemia diagnosis
The 24,25-dihydroxyvitamin D [24,25(OH)2D] is a catabolite of 25-hydroxyvitamin D [25(OH)D]. This transformation is performed by 1,25-hydroxyvitamin D 24-hydroxylase (or 24-hydroxylase, encoded by the CYP24A1 gene). Mutations in CYP24A1 can lead to severe diseases such as idiopathic infantile hypercalcemia (IIH). Explorations of hypercalcemia with suppressed parathyroid hormone levels and normal or high phosphatemia should now include 24,25(OH)2D determination to exclude CYP24A1 mutations. 24,25(OH)2D and the vitamin D metabolite ratio (VMR) [i.e. 25(OH)D/24,25(OH)2D] are now considered as new biomarkers for the assessment of functional vitamin D deficiency.
by L. Vranken, C. Fontaine, Prof. JC. Souberbielle and Prof. E. Cavalier
Vitamin D metabolism
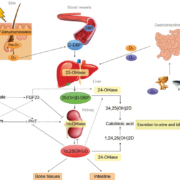
Nowadays, there is an increased focus on the vitamin D and its benefits on health maintenance and disease prevention. Vitamin D is mainly produced following skin exposure to UVB rays. Additionally, it is found in several foods, such as oily fish, mushrooms and egg yolk. Vitamin D is considered as a pro-hormone owing to the fact that its production in the skin from 7-dehydrocholesterol could be sufficient when the sun exposure is adequate. Two forms of vitamin D coexist: vitamin D2 produced by vegetables, and vitamin D3 produced by animals and humans [2, 8]. After its synthesis in the skin or its intestinal absorption, this liposoluble vitamin is transported to the liver where it is hydroxylated by vitamin D 25-hydroxylase (or 25-hydroxylase, encoded by the CYP2R1 gene) to form 25-hydroxyvitamin D [25(OH)D]. This hydroxylation is very poorly regulated and, therefore, most of the circulating vitamin D will be metabolized into 25(OH)D. 25(OH)D is then transported to the kidney by a specific protein carrier [vitamin D binding protein (DBP)], and to a lesser extent by albumin, where it is hydroxylated by 25-hydroxyvitamin D-1 alpha hydroxylase (or 1α-hydroxylase, encoded by the CYP27B1 gene) on the carbon in position 1 to form the most active metabolite, 1,25-dihydroxy-vitamin D [1,25(OH)2D]. This transformation is strictly regulated, notably by the parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23) and 1,25(OH)2D itself (Fig. 1). The major role of vitamin D is the maintenance of calcium homeostasis, by acting on the vitamin D receptor (VDR). Calcium regulation is very complex and not fully understood yet. When ionized calcium decreases, the calcium sensing receptors (CaSR) located on the surface of the parathyroid glands stimulate PTH secretion.
PTH then acts on different targets to increase serum calcium concentration: it stimulates the release of calcium (and phosphate) from bones by acting on osteoclasts through osteoblasts and the RANK/RANKL system. It also decreases calcium excretion by the kidney and stimulates 1α-hydroxylase to produce 1,25(OH)2D which, in turn, acts on the VDR of intestinal cells to produce calbindin 9k, TRPV6 and the NCX1 Ca/Na exchanger increasing intestinal absorption of calcium. The resulting increase of calcium levels inhibits CaSR-stimulated PTH production, but 1,25(OH)2D also acts as a feedback loop to stop PTH synthesis. 1,25(OH)2D finally acts on the VDR of the FGF23 gene to stimulate FGF23 production. In turn, FGF23, which is the most potent phosphaturic hormone (it inhibits Npt2a and Npt2c sodium-dependent phosphate co-transporters in the proximal renal tubule), blocks the activity of 1α-hydroxylase and stimulates 24-hydroxylase which leads to 25(OH)D and 1,25(OH)2D catabolism (Fig. 2).
24-Hydroxylase is a key enzyme that catalyses the inactivation of svitamin D. It is expressed in most vitamin D target cells and is also stimulated by 1,25(OH)2D, which hence regulates its own metabolism, therefore protecting against hypercalcemia and limiting the levels of 1,25(OH)2D in cells [1]. Production of 1,24,25(OH)3D and 24,25(OH)2D is the first step of a five-step pathway that transforms vitamin D in a more hydrophilic compound, calcitroic acid, and allows its excretion in urine and in bile [2–6, 8]. 24,25(OH)2D has a half-life of approximately 7 days and a concentration in the range of 1 to 10 ng/mL in healthy individuals.
CYP24A1 mutations
Loss-of-function mutations of the CYP24A1 gene have been identified in children presenting with idiopathic infantile hypercalcemia (IIH). These CYP24A1 gene product (24-hydroxylase) defects can be inherited as an autosomal recessive biallelic mutation. Infants present with severe hypercalcemia, suppressed PTH levels, hypercalciuria and medullary nephrocalcinosis owing to hypersensitivity to
vitamin D [4]. Indeed, there is no transformation of 25(OH)D and 1,25(OH)2D to 24,25(OH)2D and 1,24,25(OH)3D leading to a prolonged and excessive elevation of 25(OH)D and 1,25(OH)2D concentrations and an incapacity to clear them from plasma. By feedback, there will be a decrease of PTH and an increase in FGF23 concentrations (Fig. 2). These symptoms are similar to those met in vitamin D intoxication and it is important to make the distinction between these two diseases. In IIH, the vitamin D metabolite ratio (VMR), the ratio between 25(OH)D and 24,25(OH)2D, allows the differential diagnosis of 24-hydroxylase defects from vitamin D intoxication. In IIH, the VMR will be high (>50–80); that is to say high 25(OH)D with low 24,25(OH)2D, and is indicative of idiopathic hypercalcemia due to CYP24A1 gene mutations. In vitamin D intoxication, the VMR is normal because both 25(OH)D and 24,25(OH)2D are increased. Moreover, the VMR may be more accurate for revealing this mutation than 24,25(OH)2D alone because the ratio takes into consideration the circulating 25(OH)D and provides a clear distinction from a vitamin D deficiency, in which both 25(OH)D and 24,25(OH)2D are low. Indeed, if the substrate decreases, in this case 25(OH)D, the activity of 24-hydroxylase is reduced, thus the production of 24,25(OH)2D is low [4]. These genetic mutations indicate that vitamin D supplementation in children could be potentially deleterious. In these children, vitamin D supplementation must be eliminated. Indeed, they may have failure to thrive, vomiting, dehydratation, spikes of fever and nephrocalcinosis. Supplementation of mothers with 24-hydroxylase defects during pregnancy could lead to hypercalcemia associated with prematurity and intra-uterine growth retardation. Treatment of IIH encompasses the avoidance of sun and calcium- and vitamin D-rich foods. However, recently, it has been shown that isoniazid could induce the cytochrome P450 3A4, which is another vitamin D degradation pathway [9].
Thereafter, Molin et al. found that CYP24A1 gene mutations are frequently associated with renal complications including renal failure, nephrolithiasis and nephrocalcinosis. Also, they suggest that this loss-of-function of 24-hydroxylase is the most recently elucidated cause of hypercalcemia after parathyroid hypercalcemia, vitamin D intoxication and poorly regulated 1α-hydroxylation [3]. They have described patients with CYP24A1 heterozygous mutations, mostly asymptomatic, implying a hypothesis of an autosomal-dominant trait from which clinical consequences would vary throughout life and where hypercalcemia would appear only when vitamin D intakes are excessive.
Less severe mutations have been observed in patients with moderate hypercalcemia and inappropriately low PTH (<20 pg/mL). Those patients are likely to develop nephrolithiasis. 24,25(OH)2D evaluation should be done on subjects with hypercalcemia and low PTH, especially as they suffer from nephrolithiasis. Not all the mutations have been discovered yet and further genetic studies are required. Moreover Ginsberg et al. found that lower 24,25(OH)2D concentrations and lower VMR are associated with increased hip-fracture risk in community-living older men and women. They also noticed that higher 24,25(OH)2D concentrations were associated with higher bone mineral density (BMD), whereas VMR was not. Additionally, 1,25(OH)2D concentrations were not associated with BMD, consistent with previous studies in older adults [1]. In addition to catabolism, many studies tend to demonstrate that the 24,25(OH)2D may have its own biological activity in vitro in calcium regulation [5, 6]. Finally, recent studies suggest that the assessment of 24,25(OH)2D or the assessment of the VMR could better reflect the activity of the VDR and could be used as an index of vitamin D clearance [1, 3, 4]. The VMR may have the advantage of being uninfluenced by DBP concentrations, which affects both the numerator and denominator of the ratio.
Vitamin D metabolite evaluation
Quantitative evaluation of 24,25(OH)2D is complicated by its presence at low concentrations. LC-MS/MS is currently the only alternative to evaluate 24,25(OH)2D levels and has the great advantage to distinguish simultaneously the different metabolites and 25(OH)D in serum [6, 10]. The NIST (National Institute of Standards and Technology) has recently issued a new serum-matrix standard reference material [11] and Tai et al. published a reference measurement procedure for the determination of 24,25(OH)2D in human serum using isotope-dilution LC-MS/MS [10].
Conclusion
In conclusion, the assessment of 25(OH)D alone is not always enough. 24,25(OH)2D and VMR are other available tools to help for the diagnosis and the monitoring of abnormalities in phosphocalcic metabolism. The drawback is that it requires the determination of vitamin D metabolites by LC-MS/MS, and very few laboratories perform this determination [only 10 labs participate in the 24,25(OH)2D proficiency testing provided by the Vitamin D External Quality Assessment Scheme (DEQAS)]. Collaboration with a reference lab may be a good compromise. It is important to be aware of hypercalcemia caused by CYP24A1 mutants and their consequences on health. Further studies will be needed to explore the others mutations of CYP24A1 and the potential biological activity of 24,25(OH)2D in vivo.
References
1. Ginsberg C, Katz R, de Boer IH, Kestenbaum BR, Chonchol M, Shlipak MG, Sarnak MJ, Hoofnagle AN, Rifkin DE, et al. The 24,25 to 25-hydroxyvitamin D ratio and fracture risk in older adults: the cardiovascular health study. Bone 2018; 107: 124–130.
2. Vranken L, Emonts P, Bruyère O, Cavalier E. Prévalence de l’hypovitaminose D chez la femme enceinte: quelle est la situation en région liégeoise? Revue Médicale de Liège 2018; 73 (1): 10–16 [in French].
3. Molin A, Baudoin R, Kaufmann M, Souberbielle JC, Ryckewaert A, Vantyghem MC, Eckart P, Bacchetta J, Deschenes G, et al. CYP24A1 mutations in a cohort of hypercalcemic patients: evidence for a recessive trait. J Clin Endocrinol Metab 2015; 100(10): E1343–E1352.
4. Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Bröking E, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 2011; 365(5): 410–421.
5. Van Leeuwen JPTM, an den Bemd GJCM, van Driel M, Buurman CJ, Pols HAP. 24,25-Dihydroxyvitamin D3 and bone metabolism. Steroids 2011; 66: 375–380.
6. Wagner D, Hanwell HE, Schnabl K, Yazdanpanah M, Kimball S, Fu L, Sidhom G, Rousseau D, Cole DEC, Vieth R. The ratio of serum 24,25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 is predictive of 25-hydroxyvitamin D3 response to vitamin D3 supplementation. J Steroid Biochem Mol Biol 2011; 126: 72–77.
7. Lu X, Chen Z, Mylarapu N, Watsky MA. Effects of 1,25 and 24,25 vitamin D on corneal epithelial proliferation, migration and vitamin D metabolizing and catabolizing enzymes. Sci Rep 2017; 16951: 1–12.
8. Bikle DD. Vitamin D and bone. Curr Osteoporos Rep 2012; 10(2): 151–159.
9. An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway. Wang Z, Lin YS, Zheng XE, Senn T, Hashizume T, Scian M, Dickmann LJ, Nelson SD, Baillie TA, et al. Mol Pharmacol 2012; 81(4): 498–509.
10. Tai SSC, Nelson MA. Candidate reference measurement procedure for the determination of (24R),25-dihydroxyvitamin D3 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem 2015; 87: 7964–7970.
11. Tai SS, Nelson MA, Bedner M, Lang BE, Phinney KW, Sander LC, Yen JH, Betz JM, Sempos CT, Wise SA. Development of standard reference material (SRM) 2973 vitamin D metabolites in frozen human serum (high level). J AOAC Int 2017; 100(5): 1294–1303.
The authors
Laura Vranken1, Corentin Fontaine1, Jean-Claude Souberbielle2 PhD, Etienne Cavalier1 PhD
1Clinical Chemistry, University of Liège, CHU Sart-Tilman, Belgium
2Service des Explorations Fonctionnelles, Hôpital Necker-Enfants Malades, Paris, France
*Corresponding author
E-mail: Laura.vranken@chuliege.be