Lifespin to collaborate wth Biobank Graz to expand database of metabolic profiles for novel oncology diagnostics platform
Regensburg, Germany-based Lifespin, has entered a collaborative agreement with Biobank Graz and the Clinical Division of Oncology of the Medical University of Graz to expand its database of metabolic human health profiles in the field of oncology. Under the agreement, Lifespin will be able to analyze and capture the metabolic profiles from more than 25,000 longitudinal human blood samples involving approximately 4,800 oncological patients.
Lifespin is a deep data company that is mapping the metabolic profiles of human health. The company is developing a groundbreaking, automated, highly scalable, and cost-effective next generation health diagnostics platform that utilizes artificial intelligence to quantitatively identify variations in a person’s metabolism from the healthy baseline of relevant peer groups in its database. These variations can be linked to diseases with high sensitivity and specificity. Lifespin combines biology, deep data, artificial intelligence, and cloud technologies to enable digital metabolic insights for precision diagnostics and personalized disease management.
Lifespin has already gathered a significant database of health profiles in oncology. “This substantial addition of about 25,000 longitudinal oncological patient samples to our already significant and proprietary database will enable us to further advance our algorithms to help clinicians detect diseases earlier, and more reliably customize treatment and monitoring options for patients using metabolomics as a valuable guide,” said Dr Ali Tinazli, CEO of Lifespin.
“The extraordinary depth of the data we will be able to analyze as a result of this important agreement will not only allow us to gain further insights to detect malignant diseases earlier, but will also provide valuable information on metabolic changes in cancer patients overtime, notably to better understand individual
treatment response and, post treatment and in remission, to improve patient monitoring and follow-up,” Dr Tinazli added.
Prof. Philipp Jost, Director of the Clinical Division of Oncology at the Medical University of Graz and member of the steering board of the Biobank Graz, said: “Our scientific cooperation with Lifespin supports our central goal as physician scientists to apply translational cancer research towards clinical diagnostics and treatment implementations. The Lifespin platform enables us to conduct metabolic analysis of samples from cancer patients at unprecedented depth and speed, providing a very important steppingstone for detecting cancer earlier and monitoring treatment responses more efficiently.”
Earlier this year, to advance its research and development opportunities, and in preparation of its market entry, Lifespin announced the establishment of an U.S. subsidiary, Lifespin, Inc., based in Boston, Massachusetts.
Commercial products in the regulated space will be software algorithms for health testing, scalable via the cloud as Software-as-a-Service. Development efforts are ongoing for a suite of products in the field of oncology to further extend Lifespin’s product portfolio over the coming years to be made available to participating providers and laboratories.
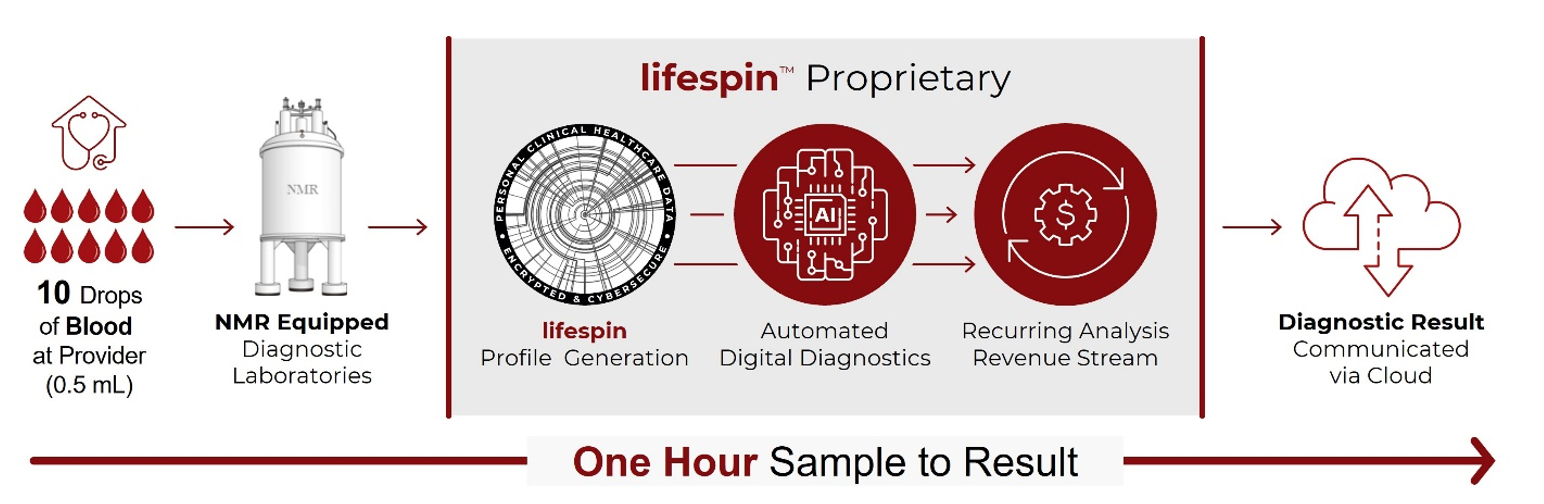
Lifespin’s proprietary diagnostic technology platform can quantitatively capture individual metabolisms, i.e., up to hundreds of metabolite concentrations with a single Nuclear Magnetic Resonance measurement. Utilizing its proprietary technology, Lifespin is performing quantitative in-house measurements of metabolomes, digitizing metabolic profiles that include billions of metabolic relationships. These digital metabolic profiles allow systematic mapping across various health conditions and will enable differential diagnosis and early detection of health conditions, staging of diseases, monitoring of treatment success and personalized medicine.
Lifespin’s first regulatory approved Software-as-IVD will be for the early differential diagnosis of Multiple Sclerosis and is expected in Europe this year, and thereafter in the United States. Additional tests for the detection of neurological, cancer, and inflamm