Euroimmun releases fast PCR-based assay for detection of SARS-CoV-2
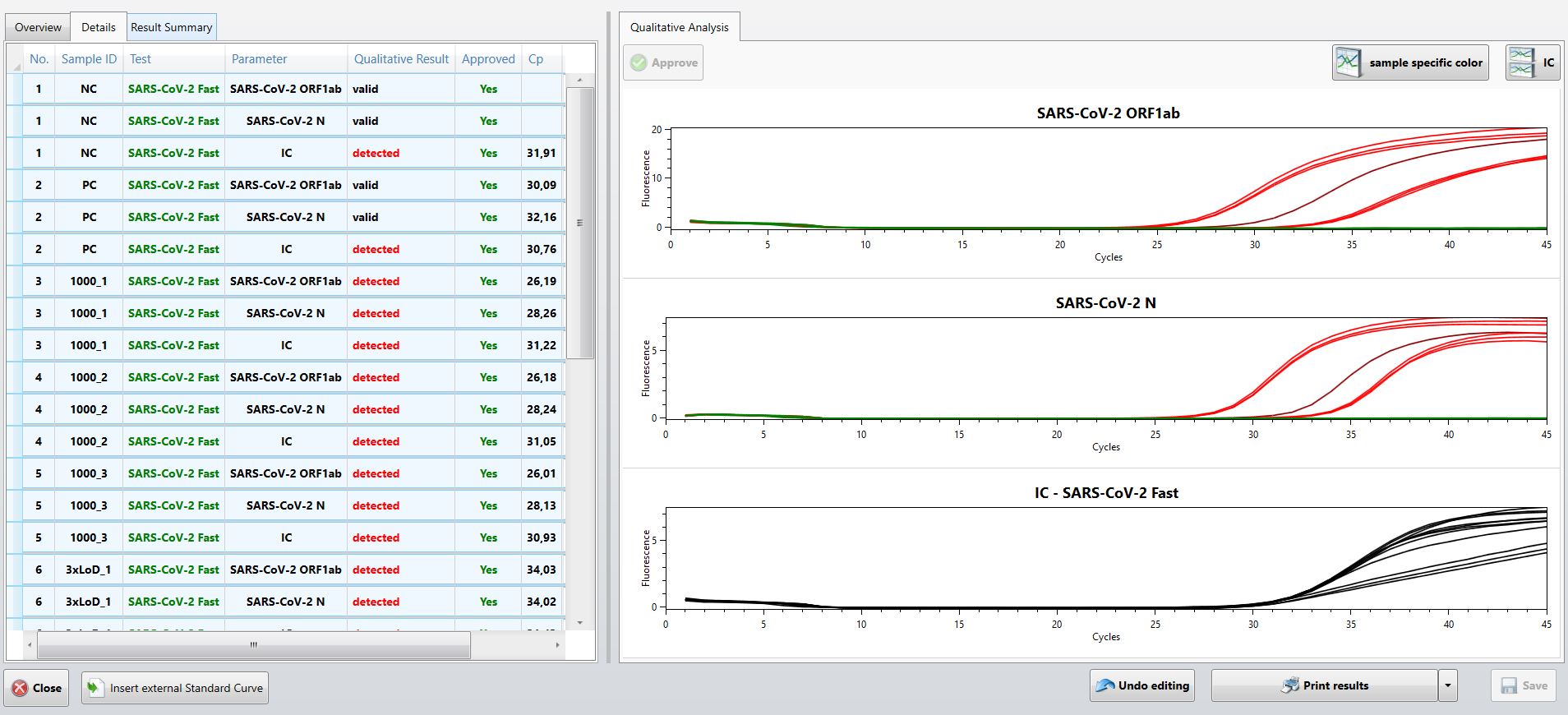
The new EURORealTime SARS-CoV-2 Fast is a CE-marked assay for PCR-based specific detection of SARS-CoV-2 from nasopharyngeal swabs in as little as 45 minutes, depending on the device used.
Reverse transcription and real-time PCR are performed in one step, so that only one reaction is required per sample. The test provides high sensitivity due to simultaneous detection of two defined target sequences in the ORF1ab and N genes of the viral genome. The detection of the target sequences is carried out in two separate fluorescence channels. The test contains an internal amplification control, which serves as an inhibition and extraction control. A SARS-CoV-2 positive control provided in the test kit is analysed as an external control in every test run ensuring dependable results. Complementary EURORealTime Analysis software provides fully automated and standardised evaluation and documentation of results, including all control results. The software also provides full guidance through the individual works steps, ensuring a simple and error-free test procedure. Comprehensive automation options for sample preparation, PCR setup and PCR performance are available.