Global Access Diagnostics reintroduces IT-Leish test for visceral leishmaniasis
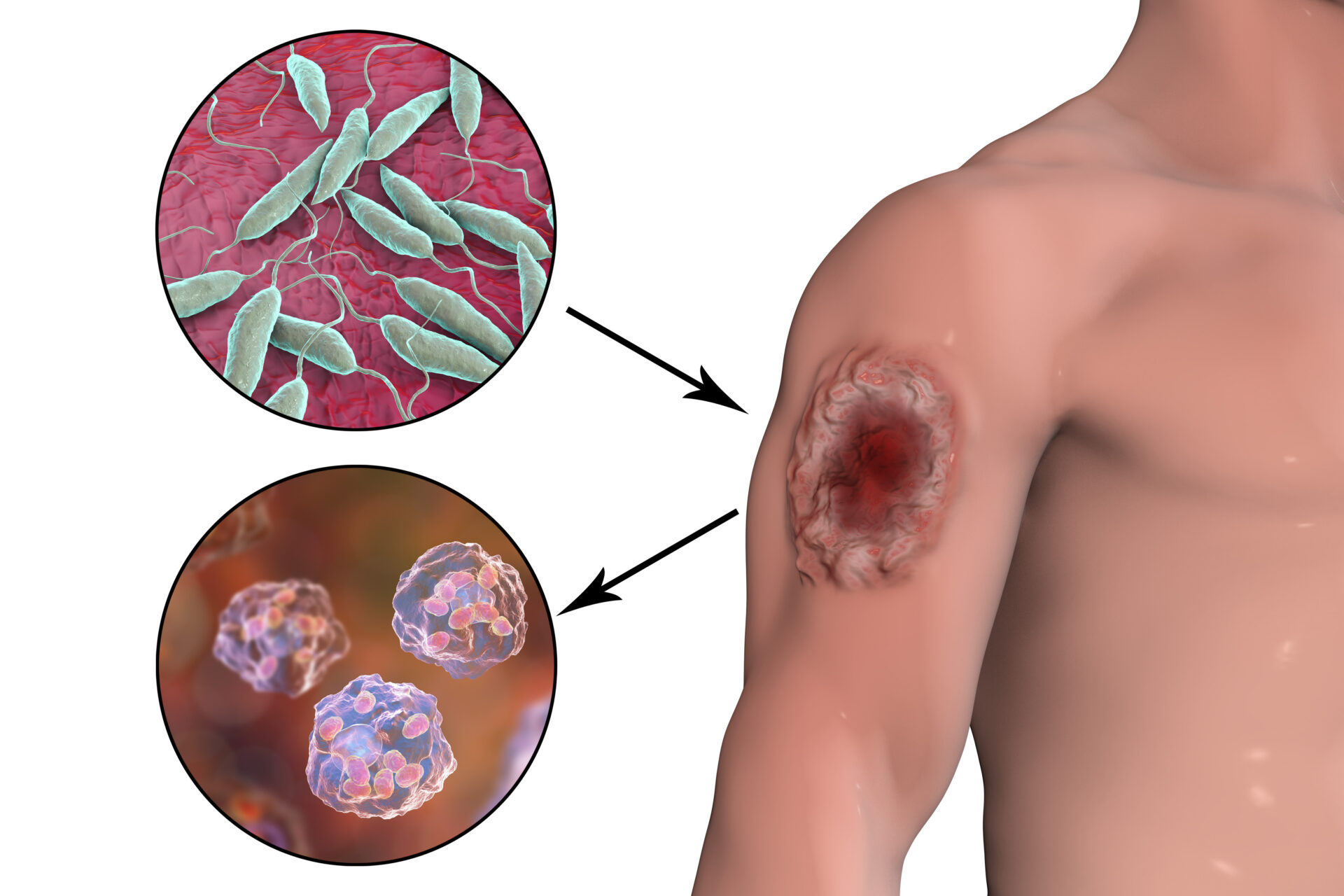
Global Access Diagnostics (GADx), a social enterprise prioritising equitable access to diagnostics, has introduced IT-Leish, a rapid diagnostic test (RDT) for visceral leishmaniasis (VL). The company acquired the manufacturing rights for IT-Leish from Bio-Rad in September 2022 to prevent its withdrawal from the market and has since ensured the regulatory requirements have been met for reintroduction to the market.
IT-Leish is a high-performance, immuno-chromatographic RDT that is used for the diagnosis of VL, a life-threatening neglected tropical disease (NTD) transmitted by phlebotomine sandflies. Early VL diagnosis is a vital component of controlling the disease, which is considered fatal if not treated rapidly. IT-Leish is a trusted RDT, taking 20 minutes to generate results. It provides reliable and accurate confirmation of clinically suspected cases of VL to enable early diagnosis, reducing disease fatality.
In January 2023, the WHO announced the development of a strategic plan to review the current epidemiological situation in East Africa, with regional and country-level elimination targets for 2023-2027. As part of this and the WHO’s wider 2021-2030 road map for NTDs, plans will be drawn to ensure long-term and sustainable financing and the procurement of medical supplies, including first-line diagnostics. By harnessing its expertise in lateral flow development and manufacturing, GADx has ensured IT-Leish is fit for regulatory purposes and available on the market as UKCA marked to support these critical campaigns.
For more information visit: www.globalaccessdx.com
Digital issue: Please click here for more information