Dual role of alpha-synuclein in calcium regulation may have major implications for Parkinson’s disease process
The protein alpha-synuclein (aSN) has long been known as a main cause in Parkinson’s disease and Lewy Body Dementia, for example, when it forms lumpy protein aggregates that destroy cell function – but aSN in its natural form, without clumping, has not had a concrete, known function.
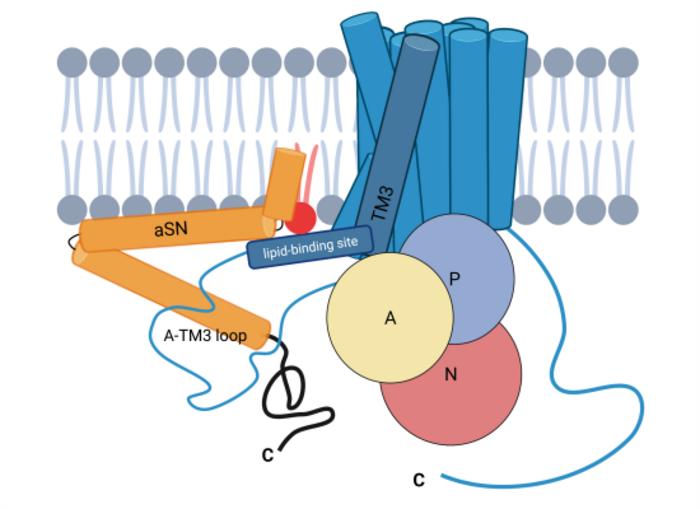
An international research team – led by Poul Nissen and Poul Henning Jensen from DANDRITE – has now demonstrated that natural aSN is an important activator of an essential calcium pump in the cell membrane.
Previously, the protein calmodulin was the only known activation factor of PMCA, but the researchers now add another important player and show that the activation of PMCA by aSN takes place in negatively charged membrane environments, complementary to calmodulin, which acts in neutral and possibly positively charged lipid environments.
Nissen explains: “The activation appears to be of particular importance in the presynaptic area of neurons, which is responsible for transmitting signals in the brain’s neural network. It is known that aSN accumulates in the presynaptic compartment, and our study shows, for the first time, that aSN and PMCA appear together in this area. In addition, we show that aSN stimulates calcium excretion from neurons, and not least that aSN significantly increases the activity of the PMCA.”
In addition, the researchers show by mathematical network modelling that PMCA activation likely to be of key importance for the calcium homeostasis, when neurons signal repeatedly, and calcium therefore enters the cell constantly. aSN’s activation of PMCA thus prevents a build up of calcium that would be toxic to the cell.
Henning Jensen elaborates: “Our laboratory has previously shown that early clump stages of aSN activate another calcium pump, SERCA, which sits in an internal organelle of the cell (endoplasmic reticulum), but SERCA is not activated much by soluble aSN. Oppositely, PMCA is activated a lot by soluble aSN and almost not by clumped aSN.”
Calcium regulation by PMCA and SERCA is essential for all cells, not least neurons, so if a pathological condition is associated with transitioning from natural aSN to clumped aSN, there is also a shift in aSN activation from PMCA to SERCA, and calcium regulation is thus strongly affected. It changes a multitude of processes, and ultimately leads to cell death.
New research from Aarhus University with international collaborations has revealed that natural aSN activates an important calcium pump in the cell membrane.
Credit: Antoni Kowalski
The crucial role of calcium regulation
Calcium regulation is fundamental to the function of all cells, including neurons. It plays a crucial role in signal transmission, particularly in the presynaptic area where native aSN is typically found. When aSN clumps, this balance is therefore shifted from PMCA- to SERCA-activated calcium regulation, and this changes the calcium balance in cells. This is thought to take place early in diseases such as Parkinson’s.
This newfound understanding of aSN’s dual role in calcium regulation could have profound implications for unravelling the early disease processes, especially in Parkinson’s disease. By identifying the shift from natural aSN activation of PMCA to clumped aSN activation of SERCA, researchers can gain insights into the mechanisms that underlie the onset and progression of neuro-degenerative conditions.
Future research and therapeutic potential
As researchers continue to explore the intricate relationship between aSN and calcium pumps, it opens the door to potential diagnostic and therapeutic strategies aimed at exploring and mitigating the early disruptions in calcium regulation.
The discovery of natural aSN’s role in calcium pump activation represents an important step towards understanding the complex biology of neurodegenerative diseases and how we might be able to cure them.
DANDRITE is currently building strong positions on studies of new insights into synaptic calcium signalling in both the healthy state and under Parkinson’s Disease conditions. For example, new DANDRITE group leader Chao Sun has established a research programme to locate and track the individual molecular players in synapses that regulate calcium signalling. The goal is to gain a much more precise and operational understanding of early, crucial tipping points in neurodegenerative disorders and how to steer around them.
The research results were published in The EMBO Journal.
https://doi.org/10.15252/embj.2022111122