Ventilator-associated pneumonia Challenges of diagnosis
Critically ill patients who cannot spontaneously breathe are kept alive in the Intensive Care Unit by mechanical ventilation which delivers oxygen and removes carbon dioxide via an endotracheal tube. Ventilator-associated pneumonia (VAP) is an added burden for these patients, and their outcomes are improved if it can be diagnosed and treated early. However, diagnosis of VAP is not necessarily straightforward; CLI chatted to Dr Cyril Chacko (Consultant in Critical Care Medicine and Anaesthesia, Royal Wolverhampton Hospitals NHS Trust, Wolverhampton, UK) to find out more about VAP, how it is diagnosed and the pitfalls and challenges of diagnosis.
What is ventilator-associated pneumonia?
Ventilator-associated pneumonia (VAP) is a nosocomial infection of the lung parenchyma. It normally develops 8 hours after intubation and mechanical ventilation. Now, because of the discrepancy associated with the diagnostic criteria of VAP, there is a range of the reported incidence of VAP in the literature of between 20 and 40%. One of the points that increases the discrepancy in the reported incidence is the timing: most current literature would say that 48 hours – whereas some says 72 hours – after intubation is when you would describe an incidence of VAP.
One of the other factors that raises the discrepancy in the incidence of VAP is the criteria for diagnosis, which again varies between different institutions, where they would say it would have to have a clinical, radiological and microbiological criteria for diagnosis, prior to making a full diagnosis of VAP. Also, there can be variability between clinicians about the clinical aspect of diagnosis of VAP, which again contributes to the discrepancy in reported incidence rates.
The clinical criteria for VAP are a new onset of increased sputum secretions, and/or a change in characteristics and quantity of the sputum secretions; new onset of unexplained leukocytosis or leukopenia; or an indication of a new infection focus, such as a temperature rise, which has not been explained by any other source of infection in those groups. This makes it sound very black and white, but, unfortunately – particularly in critical care – there is a significant other group of pathologies that can lead to a temperature rise or
new leukocytosis or leukopenia: drugs being one of the most common cause of those symptoms. So that is why there are radiological and microbiological criteria for VAP also – this helps to get a more conclusive diagnosis.
VAP has a significant burden on critically ill patients, because it is one of those added complications that are associated with the support that we need to deliver to critical care patients. When we have a patient who is critically ill and we have to intubate and ventilate them, this leads to the loss of the natural, protective, upper airway that is present in the normal patient, as well as the mucociliary function of the respiratory system, which makes a patient more prone to nosocomial infections such as VAP. Not only does VAP place a significant burden on patients in the Intensive Care Unit (ICU), it also increases the morbidity and mortality rates of patients in the ICU by about 20–70%, depending on the literature that you read. Also, even in patients that survive, their length of ICU stay is prolonged by about 7 or 8 days, which leads to an increased burden on ICU resources.
So, VAP is a very prevalent problem, and it is associated with increased morbidity and mortality. Another problem associated with VAP is the development of multidrug-resistant (MDR) organisms in intensive care because of the increased use of antibiotics to treat VAP, which leads to further problems for patients in intensive care.
Mechanical ventilation in the Intensive Care Unit
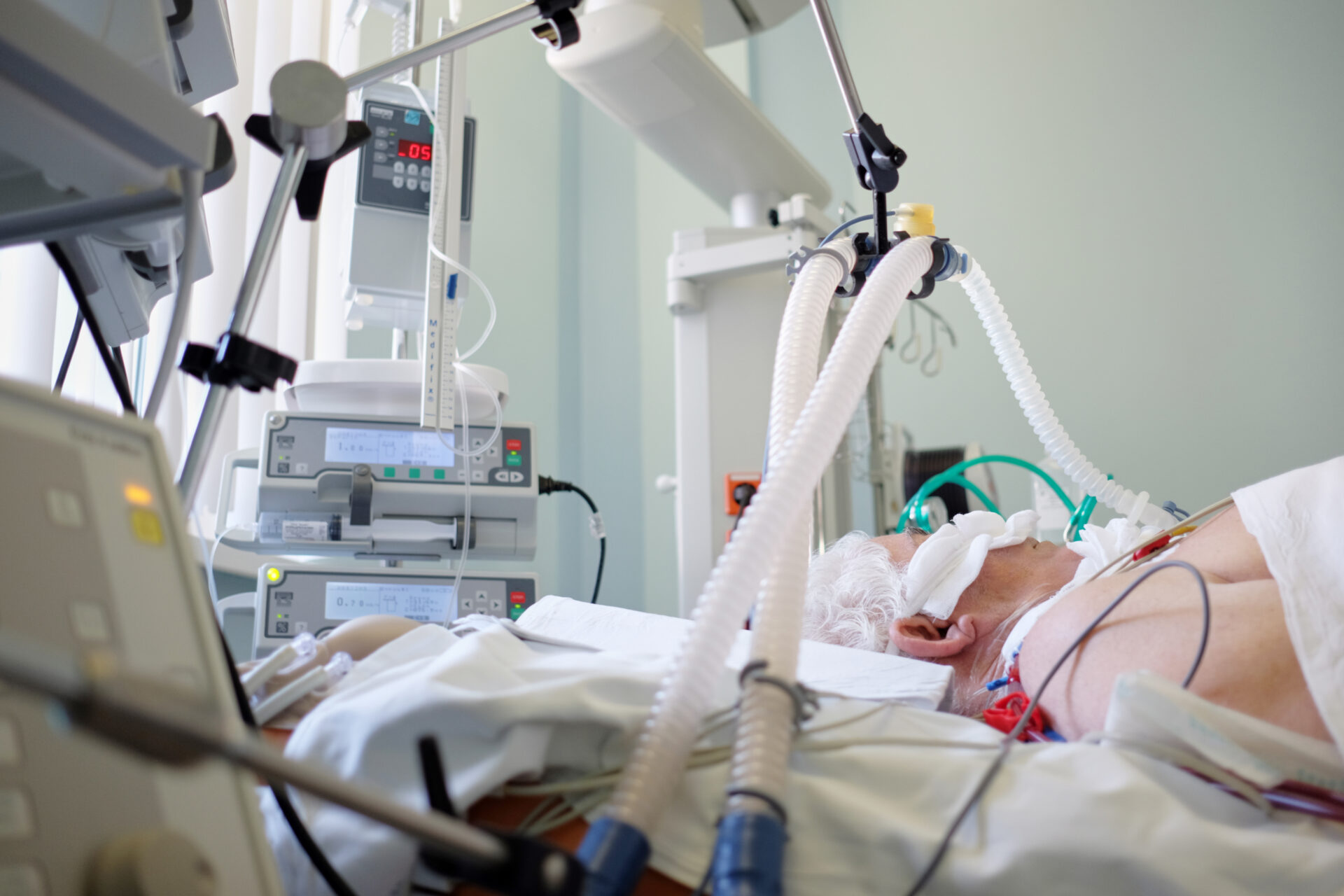
Invasive mechanical ventilation is necessary for seriously ill patients whose spontaneous breathing is inadequate to maintain life. Oxygen is delivered to (and carbon dioxide removed from) the lungs via an endotracheal tube. This and the presence of other monitoring equipment makes access to and moving the patient challenging. (Adobe stock)
What factors contribute to the causes of VAP?
The common risk factors for patients to go on to develop VAP are those who have intubation and mechanical ventilation, patients who are paralysed and are sedated, and patients that are in a semi-recumbent position, because they do not have their upper airway and other factors that protect patients who are breathing spontaneously. When you have sedation and paralysis, you lose your normal protective reflexes of coughing and moving the secretions away from the lungs, which leads to a higher risk of patients developing VAP. The semi-recumbent position, which allows postural drainage – the term that I like is that there is a ‘gravitational gradient’ – so that the mucus and respiratory secretions pool down into areas of the lung from where you can not clear it, even if you are fit and healthy and walking around. That area of the lung then is prone to developing infection, and the patient is at increased risk of developing VAP. On top of this, what we have is intubation of the lung, and what develops in the endotracheal tube is a biofilm, which is becoming increasingly associated with nosocomial infection – in the same way that a biofilm is associated with increased risk of catheter-related bloodstream infections. The biofilm on the endotracheal tube can become dislodged or transmitted down into the lung parenchyma when you have movement or suctioning, or through other effects of the patient. Additionally, there is a suction tube around the endotracheal tube and the micro-secretions accumulate around the top of the cuff and slowly percolate down into the lung parenchyma, and this leads to the patient being more prone to infection. Again, the other thing that also affects the incidence of VAP is the innate response of the immune system against invading pathogens, such as the alveolar macrophages and the neutrophils and the inflammatory response, which leads to more inflamed areas in the body.
Is there a histological definition of VAP?
The gold standard definition for the histological diagnosis VAP is the presence of consolidation of leukocyte accumulation in the bronchioles and alveoli. Now, there are four stages of infection in VAP, histologically. The first stage is bronchiolitis, which is characterized by blood clotting within the bronchioles and the bronchiolar wall. The second stage is focal bronchopneumonia, with neutrophil infiltration into the terminal bronchioles and alveoli. Third, is confluent bronchopneumonia, with focal infiltrates extending into adjacent lobes. The final, fourth stage, is the development of a lung abscess, with extension of the bronchopneumonia and additional tissue necrosis.
Again, the histology suggests why there are challenges in diagnosis, as there is a wide period of time across the pathogenesis of VAP, with a very heterogeneous presentation over that period of time. We have to bear in mind that we have to obtain the right specimens. Usually this is a low lobe infection, so the possibility of getting a positive sample might be compromised. Obtaining the right sample from deep in the lung is challenging, particularly from patients who have a significant requirement for oxygen and high airway pressures.
How is VAP normally diagnosed and what are the difficulties of diagnosis?
The diagnosis of VAP is based on the clinical, radiological and microbiological criteria.
Clinical criteria
For the clinical criteria, you look for new onset of cough, sputum production (both increase in quantity and change in quality of the sputum), and tachypnoea and hypoxia. In the ICU, we like our numbers a lot, so if there is a sudden deterioration in the change in P/F ratio – the ratio of the partial pressure of arterial blood oxygen (PaO2) to the fraction of inspired oxygen (FiO2) – we would start to consider VAP. Again, taking into consideration the patient’s history and previous risk factors, such a being a previous smoker, has underlying respiratory disease, etc, then we would consider this patient to be at a higher risk of developing VAP. Now, sputum is a useful marker, we check the sputum quantities and qualities against different scales, and we have to keep an eye on the trend right through the patient’s stay in ICU, so there are ways that we can try to overcome the diagnostic challenge to a certain extent.
Radiological criteria
So, these clinical indicators would raise our suspicions that this patient has VAP, and then we would go on to the diagnostic tests. Radiologically, the main modalities that we use for VAP diagnosis are chest X-ray, CT scan and ultrasound, all of which have their pros and cons.
Chest X-ray
We usually start with a chest X-ray in the UK, primarily, because it is easy to do, well tolerated by the patient and easy to interpret. However, although the sensitivity is around 90%, the specificity is very low at around 26%. Also, we are looking at a three-dimensional object on a two-dimensional view, so miss a lot of pathology that way.
Lung ultrasound
Lung ultrasound is a modality that is being used more and more in the UK. However, it is quite challenging to do lung ultrasound on a patient in intensive care, because often patients are significantly overweight, or you might have a patient who has a pneumothorax or subcutaneous emphysema, through which ultrasound can not penetrate. It is a modality with a high specificity and sensitivity, and the inter-observer variability is quite low. It is an up-and-coming tool that is being used a lot more in the intensive care setting across the globe as a tool for VAP diagnosis and other pathologies in intensive care.
CT scan
The CT scan is really a gold standard investigation for VAP. However, actually doing the CT scan is associated with a lot of problems. First, you have to transfer the patient to the CT
scanner and this transfer is associated with about 30% increase of morbidity. Additionally, there is a high radiation load on the patient, and finally getting repeated CT scans for a patient might be challenging logistically for most organizations. However, it is the gold standard of diagnosis for VAP.
Microbiological criteria
The third aspect of diagnosis is microbiological, and the challenge is how do we get the sample. There are two ways we can do it. We can do a blind aspiration, with the patient under sedation we place the suction catheter tube through the endotracheal tube as far as it can down deep into the lung and suction out the sample. These endobronchial aspirates are a tool that we use. It is simple and efficient and it is a safe technique that can be performed by any member of the ICU team. Again, the sensitivity and specificity are both around 70%; however, there are a lot of false-negative results, because a lot of these patients are already on antibiotics and an aspirate can lead to a negative culture, but we still have to look at the overall picture of the patient, including the clinical and radiological findings. The national guidelines advocate endotracheal aspiration to be used as and when we are suspicious of a diagnosis. One of the other techniques that can be used is a bronchiolar lavage, where we go in with a bronchoscope, down into the lobe, and either we can aspirate out the sputum or we can do a lavage, where a solution is injected into the lung and aspirated out for direct use in microbiological diagnosis. However, these days, techniques that involve a bronchoscope and scraping out of the sample, which is quite an invasive technique are used more rarely, as many of these patients are critically unwell. If the patient’s oxygen requirement is extremely high and the airway ventilation pressure is high, these techniques can’t be used as use of the bronchoscope releases that pressure. So although it is a very sensitive and specific test, it cannot be used in all groups of patients in intensive care.
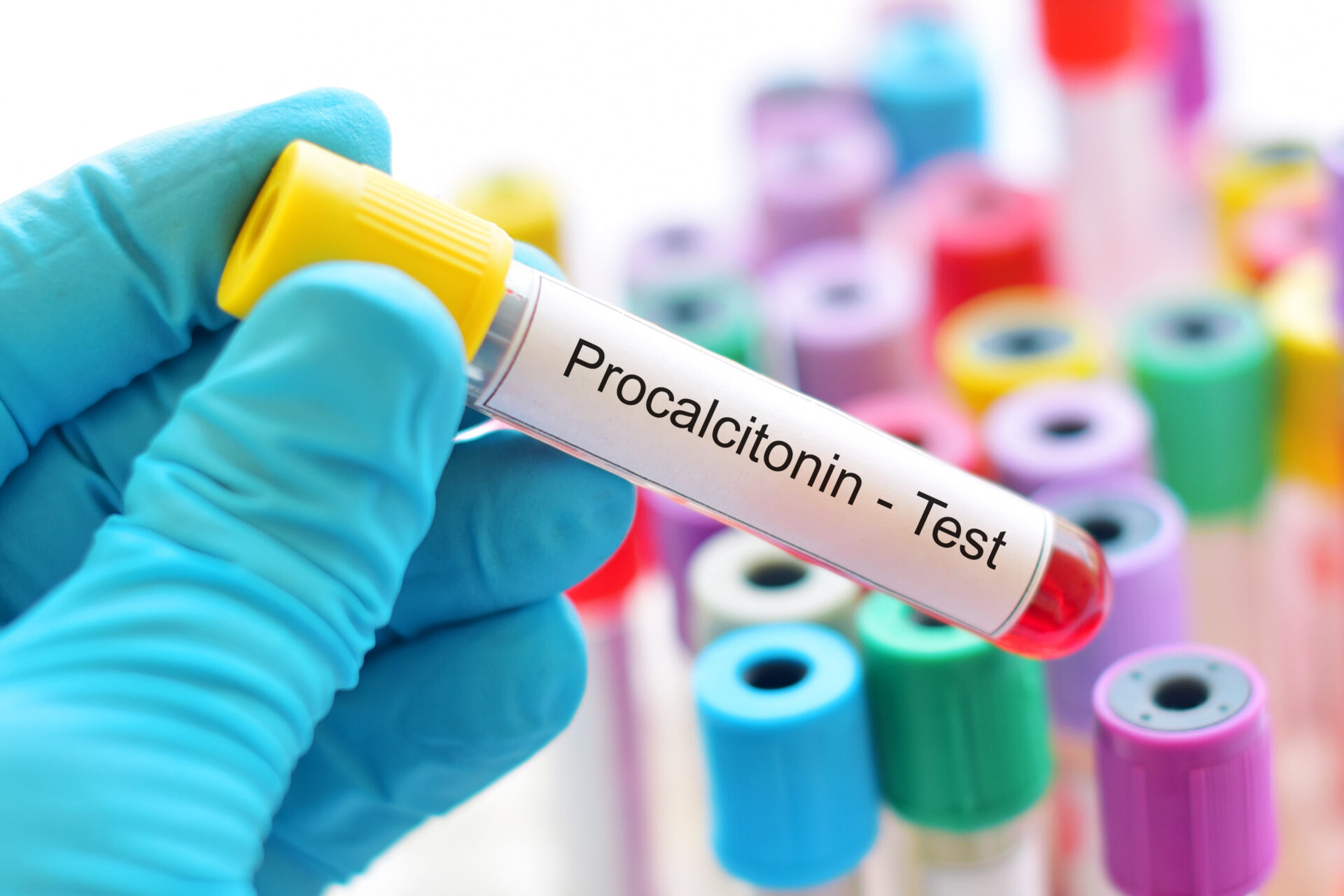
Biomarkers of inflammation can be useful to indicate a new infection (Adobe stock)
Are there any blood biomarkers that can help to diagnose VAP?
There are certain biomarkers that have been used in the diagnosis of VAP. Some of the common ones are the same as we would use for infection markers. For example, procalcitonin (PCT) is quite a sensitive marker for bacterial as well as fungal infection. It is an acute-phase product, produced by the liver, it is a prohormone secreted into serum from neuroendocrine cells in the lungs. It is not specific for VAP, it is a marker that goes up with any inflammation within the body.
The other biomarkers that we use are CRP (C-reactive protein), again it is a non-specific biological marker of inflammation, which is again synthesized in the liver and gets released in response to infection.
The sTREM-1 (soluble triggering receptors expressed on myeloid cells type 1) is a new biomarker of microbial infection. It is a member of the immunoglobulin superfamily and is strongly expressed on neutrophils and monocytes that are infiltrating cells invaded by bacteria or fungi, and it is released during inflammation.
The last group of markers that we looked at are the proinflammatory cytokines. The common ones that we have found to be increased in VAP are interleukin (IL)-8 and IL-1β,
as well as TNF-α (tumour necrosis factor alpha).
The crucial factor about all of this is that these are specific markers that increase in level in response to inflammation.
So these markers will increase in response to any inflammation or any infection, so the clinical aspect of all of this has to be taken into significant consideration. We have to think about where is the source of the infection – where do we think these acute-phase inflammatory reactants coming from. They are quite specific – e.g. PCT and sTREM – for bacterial and fungal infection, but it could be a bacterial or fungal infection in any other part of the body.
How is VAP treated?
Treatment is via antibiotics, and the data looks at the timing of the infection. Up to 92 hours is considered to be early onset VAP and after 92 hours is considered to be late onset. In early onset VAP, we often see staphylococcal or other common bacterial infection, but in late onset we start to see the more drug-resistant bacteria such as Acinetobacter and other bacteria that are more associated with multidrug resistance problems. So again, we use the timing of the onset of the infectious episode
to guide our antimicrobial policy. We start with broad-spectrum antibiotics and then narrow down our choice depending on the results of the microbiology tests and the other investigations.
What do you envisage for the future for improving diagnosis of VAP?
This is an interesting question and we can hypothesize that – one thing that will probably come into use is artificial intelligence (AI), which as the ability to analyse a multitude of data and aid us with guided decision-making. This is something that we think will help us with conditions that are difficult to diagnose, such as VAP. What would also be interesting is the machine learning that will go on along with the process of using AI – so that it becomes better the more it is used. Using technologies such as AI, would lead to early diagnosis, appropriate treatment and improvement in patient outcome.
With regard to diagnosing VAP, the main aim when treating patients with mechanical ventilation is to be aware of the possibility of VAP, to identify the deterioration in the patient early and to initiate treatment appropriately. This is key to the treatment of VAP, or any infection as such – similar to sepsis.
The interviewee
Dr Cyril Chacko MD, FRCA, FFICM, Consultant in Critical Care Medicine and Anaesthesia,
Royal Wolverhampton Hospitals NHS Trust, Wolverhampton, UK;
Associate Professor Honorary, Institute of Clinical Science, University of Birmingham.
Email: cyril.chacko@nhs.net
For further information see:
Howroyd F, Chacko C, MacDuff A et al. Ventilator-associated pneumonia: pathobiological heterogeneity and diagnostic challenges. Nat Commun 2024;15(1):6447
(https://www.nature.com/articles/s41467-024-50805-z).