Development of rapid and POC tests for viral diagnostics
It is often said that “necessity is the mother of invention”, something that often seems to happen in war situations. The COVID-19 pandemic, in many ways, felt like a war, and drove fast changes in behaviour, very quickly having us all performing lateral flow tests at home, in a way that could not have been imagined previously. Dr Jonathan Schmitz is a physician-scientist who serves as the Director of Translational Microbial Diagnostics in the Dept. of Pathology, Microbiology, & Immunology at Vanderbilt University Medical Center (Nashville, TN, USA). Before this, he served for 6 years as the Medical Director of their Molecular Infectious Diseases Laboratory, in which role he oversaw the development of diverse microbiologic testing for their health system, including implementation of SARS-CoV-2 testing. CLI has been very fortunate to chat to Dr Schmitz and learn from his insight into the development of rapid and POC tests for viral diagnostics and where it might lead.
Over the years, there has been a lot of development in the methods used for virus identification/detection – what are the different methods?
It is so broad in nature. Initially, viruses – like all microbes – were detected by culture, with the extra caveat being that you can’t culture a virus unless you have some sort of cell line or living substrate to grow them on. Viral culture naturally became the diagnostic gold standard, and in some cases remains the technical gold standard to this day. The challenge being that it’s not an easy procedure with the requirement of cell lines (or eggs or whatever that substrate may be) and it inherently requires a lot of effort. Some viruses inherently grow slowly, and some are very difficult to propagate in culture to begin with. There are some niche scenarios where diagnostic viral culture is still necessary, although it’s under the domain of more specialized reference laboratories. So, that’s the historical background.
In terms of the routine diagnostic work that is currently conducted in most medical laboratories, it really depends on the virus and the sort of infection that it causes. For viruses that cause acute infections – such as respiratory viruses and gastrointestinal viruses – it’s mainly a combination of molecular testing and antigen-based testing.
Molecular testing is a term for identification of virus-specific nucleic acid, either DNA or RNA (depending on what sort of genome the virus has), while antigen testing is essentially a catch-all term for direct immunodiagnostic detection of any other molecular structure on that virus. In antigen testing, a recombinant antibody is used to detect the viral structure, although the methods themselves are
very diverse. They include things like ELISAs (enzyme linked immunosorbent assays), chemiluminescent assays, and lateral flow assays… the latter is also known for its high ease-of-use. Often, the specific antigen target is a viral protein, but it could theoretically be a carbohydrate. In many cases, antigen testing is inherently quite rapid, although it traditionally doesn’t have quite the same analytic sensitivity as molecular testing.
Molecular testing, of course, has really come a very long way over the last over the last 20 years. In fact, it’s only been 40 years since the invention of PCR it. So, when you see where we’ve gone in essentially one generational time-span, it’s pretty amazing! Molecular viral detection most commonly involves target-based amplification, where you take an initial amount of nucleic acid – below what could give a direct empiric readout – and you amplify it to a detectable level. PCR was the massive breakthrough that then ushered in the field, although it’s important to point out that there are various other nucleic acid amplification technologies in addition to PCR. Also, with nucleic acid amplification tests, the amplification itself is typically only one part of three stages: there’s the extraction, getting the nucleic acid out of whatever environment it’s in (whether that’s a viral capsid, a bacterium, a fungus) and getting it into the biochemical milieu where you can do the second step, the amplification reaction. And then, finally, there’s the readout – how do you actually see that there’s a presence of your amplified product? Real-time PCR (or qPCR) was a major advance, where amplification and detection were combined together in a single step, and you can monitor increasing fluorescence as it happens. These techniques started to enter clinical care in the early 2000s. Although, since then, massive developments have focused on accomplishing these technologies at scale and with speed – optimizing them so that, instead of being one-off tests, automated liquid handling can allow a laboratory to perform dozens or hundreds of tests at a given time, or even allow testing outside of labs.
Beyond antigen and molecular testing, there are other modalities for viral diagnostics – again, though, it depends on the virus and clinical picture. This is especially true outside the context of acute infection, when you also consider viruses that elicit chronic infec-tions, for instance things like HIV and several hepatitis viruses. For these pathogens, traditional serology (identification of host antibodies to the pathogen) remains very important, as you’re looking for the body’s antibody response to infection. The point being that – because this is a chronic infection – it gives time for your body to mount an antibody response, so it’s a very nice screening methodology to detect infection, even in the absence of symptoms. With these chronic infections, you’re typically not testing to evaluate for acute disease, but screening to detect an occult infection that hasn’t yet manifested itself with symptoms… but that one day could be significant. So, overall, viral diagnostics at present is a combination of molecular testing, antigen testing, and – depending on the virus and the type of infection – serologic testing plays an important role too.
How have these methodologies been used and what are their limitations?
Historically, with viral culture, it gets back to the biology of the virus: culture is a more tedious process in general, there are some viruses that are very difficult to culture, and there are some viruses that we can’t – or at least don’t know how to – culture. Even for the viruses that we can culture, the obvious limitation to this method is its long turn-around time. This is very important when you’re talking about acute infections. If the test result comes back 2–3 days later – which was for many years was the classic scenario with things like influenza – you’re outside of the window for patient management decisions.
For the inherent limitations of serology, it is really about how you are trying use the test. A serologic assay can only identify a past history of infection – so, of course, it cannot provide insight on acute infections prior to the time it takes for the body to mount an antibody response. And it does not directly indicate whether a patient is still actively infected or if the virus has been cleared (or gone latent). Of course, there are some motivations for serology testing that go beyond the ability to identify past infection, per se. There are viruses for which the presence of antibodies serves as a reliable biomarker for functional immunity, even when immunity itself is mediated by factors other than just antibodies. This is the sort of classic method that we use for looking at immunity against diseases like measles, mumps and rubella. Although, even then, immunity in some individuals can wane over time, and it is the interplay between individual immunity and population-level epidemiology that keeps such viruses essentially non-existent in many areas of the world.
At-home antigen testing for SARS-CoV-2 (Adobe Stock.com)
On the flip side, there are some viruses where the body mounts an antibody response, although it does not lead to clearance of the virus. For example, we use the presence of antibodies against hepatitis C or HIV diagnostically, not as a surrogate for immunity but for the potential for active infection. Likewise, for the various human herpes viruses, serology is used to indicate viral dormancy within the body, as these pathogens are typically not cleared after initial infection. Where things can get particularly frustrating – as we saw during the pandemic – is the body’s antibody response to many acute respiratory viruses. Here, antibody levels spike following infection or vaccination, and neutralizing antibodies do contribute to immunity. But it is not a black-or-white scenario… the immune response can help minimize disease severity, critically so, but not always outright infection. Also, antibody levels (and immunity in general) decline over time – hence the motivation for boosters – although this time-course can differ from patient to patient. As a result, COVID-19 or influenza serology is not used diagnostically to assess immunity.
With antigen and nucleic acid amplification testing, key questions often come down to their relative sensitivity and the proverbial bang-for-the buck. As I mentioned before, antigen testing does not typically carry quite the same high analytic sensitivity of molecular testing. So, these assays can miss infections in some patients, depending on their individual viral burden and when testing occurs. This is the reason why a single negative antigen-test for COVID-19 does not rigorously rule out infection in an individual. At the same time, the cost and expertise required for conducting molecular testing was traditionally higher. But these considerations are also changing over time and with new technology.
That brings us on to the next question, what are the ideal characteristics of a diagnostic test?
Whether it’s an in-lab test, point of care (POC) test, or an at-home test – the term rapid diagnostic test (or RDT) is often interchanged with the latter two – the ideal characteristics, at least in a true dream situation, would be everything you could possibly want: analytic sensitivity, analytic specificity, diagnostic sensitivity and specificity, rapid turnaround time, and only costs a few cents to perform. In an ideal world, you could certainly come up with a pretty easy bucket list of performance characteristics! However, the art behind the science is where do these balance off with one another and what is that sweet spot… because, unfortunately, you can’t always have everything. Perhaps not surprisingly, greater analytic sensitivity comes at a cost, sometimes in terms of time and sometime in terms of actual price. It’s really about the interplay of these factors, and it can come back to the overarching questions of what a provider is hoping to accomplish with the results at the end of the day. I sometimes use the idea of ‘actionability’… what is the clinical action a given test can facilitate… what difference in care is it going to make? This action could involve medical management, prognosis, or the appropriate contact precautions, but it fundamentally relates to why a clinician is ordering the test in the first place.
For example, HIV screening involves a multistep process. Because we don’t want to miss a single case, the initial testing involves a combination antibody/antigen assay that intentionally sacrifices specificity for added sensitivity. Physicians and labs both know that there are occasional false-positive results, but these will be weeded out at the second level of screening, which is highly specific. These initial false-positive results are only in seen in the context of the second rule-out, so they don’t cause undue concern to patients.
On the other hand, with testing for respiratory and other acute viruses, the question often comes down to the patient’s management in the immediate future. Are there treatments available, for mitigating severe disease in vulnerable patients? Should this person quarantine or wear a mask for a few days? In this case, a test should ideally serve as a both a rule-in and a rule-out, and as rapidly as possible. But there can be a compromise when it all comprises a single-step test. As mentioned, a one-off negative antigen test for COVID-19 or influenza should not be construed as definitive evidence of no infection, because the sample may not reflect the right point in the course of disease.
Do we have anything that fits that bill?
For respiratory viruses, nucleic acid amplification testing has become the new gold standard, with its very high sensitivity and specificity. But while POC molecular diagnostics have become more widespread for several pathogens, especially in response to the pandemic, they are not yet universal and in-lab testing is required. For many patients, even in resource abundant areas of the world, same-day molecular diagnostic results are not feasible.
By contrast, lateral flow assays (LFAs) for antigen testing are such an elegant test. Their combined simplicity, speed, and low cost are fantastic, and they have already been in use for decades in other diagnostic scenarios… from rapid streptococcal assays to at-home pregnancy tests. They do have limitations, returning to the same analytic sensitivity gaps. But they still valuably impact care under many circumstances, especially when you think about the context of medical care globally. Many of us are fortunate to live in places with relatively strong health-care systems. But in locations with limited resources, where there may be no access to central laboratories – and even the ability for clinicians to follow up with patients can be compromised – the ability of these tests to provide real-time answers can make all the difference. The same analytic limitations still apply, but it’s all about context and what baseline services would otherwise be available to these patient.
As we have seen in the COVID pandemic, the other beautiful thing about LFAs is their potential adaptability for POC or at-home testing. They are simple enough that not only a physician or a nurse but the patients themselves can perform the test, with decreased concerns about the possibility of a procedural error leading to an erroneous result.
What improvements can be made to LFAs or other testing technologies?
This is a an area of tremendous interest to both biomedical engineers and molecular biologists alike. Traditional LFAs don’t amplify the target that is present – for example, with a COVID antigen assay, whatever virus is present on the collected nasal swab is diluted into the kit’s fixed buffer, a portion of which is tested on the strip. However, even if a viral antigen itself is not amplified like with PCR, there could be methods for rapid analyte concentration. One might capture all the virus from an entire specimen-collection into a smaller volume, which is then applied in total to the LFA.
There are also potential areas for assay improvement that, in one sense, draw on the history of nucleic acid testing. In the past, these assays were divided into methods that utilized signal-amplification techniques versus target amplification methodologies. For target amplification, PCR is the classic example where you’re increasing the starting DNA/RNA itself. However, there were also historically signal amplification methodologies, in which multiple probe-based annealing events could increase the observable readout from each starting nucleic acid being targeted. The notion of signal amplification for LFAs is likewise of great interest, either through improving the physical readout itself (leading to a stronger line) or the manner in which this signal is detected. For instance, is it just visualization by the naked human eye or could it be could aided with additional technology? There’s also the possibility of hybridizing
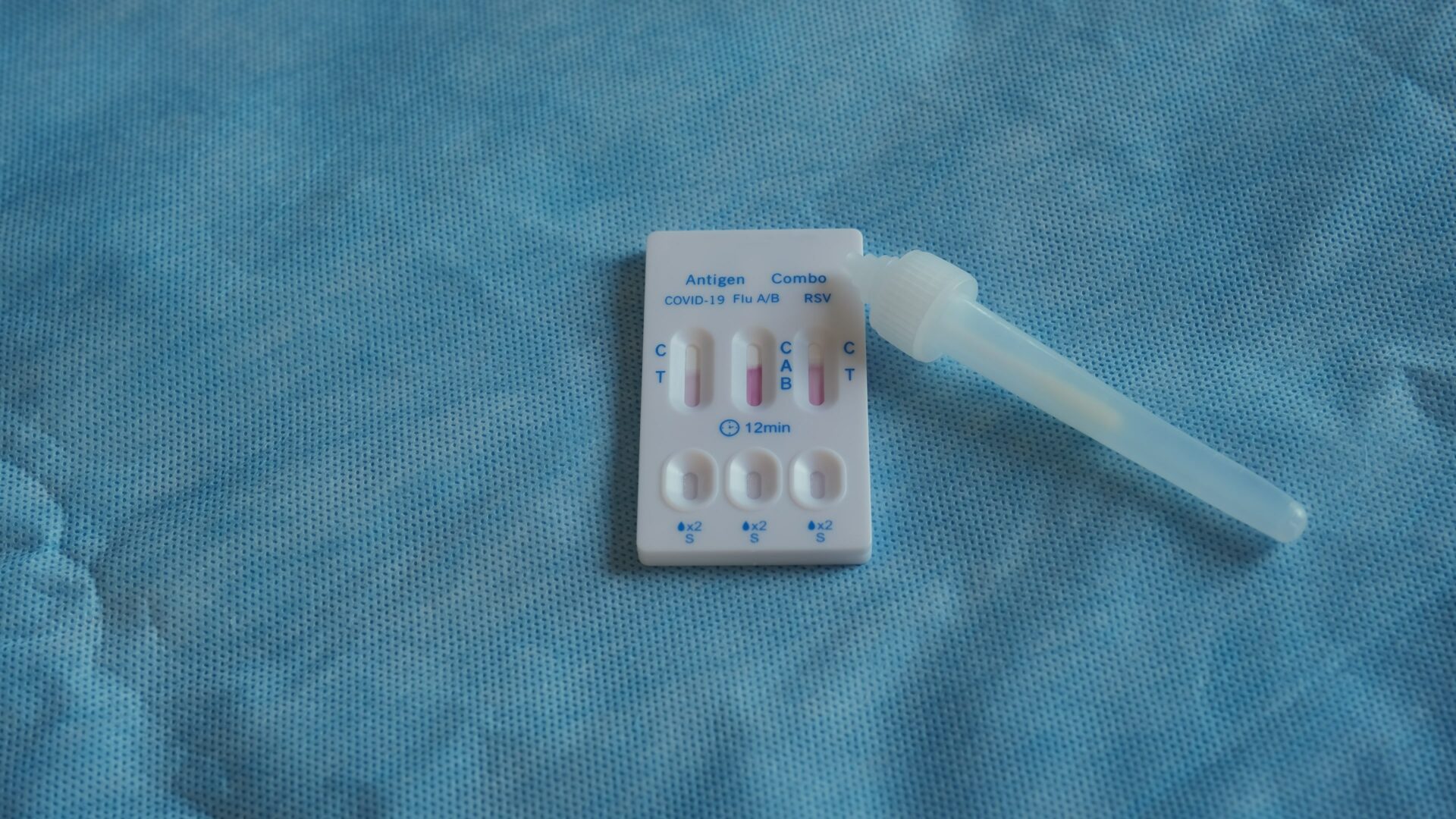
An example of a multiplex lateral flow assay: quick antigen test cassette for SARS-CoV-2, influenza (Flu)A/B and respiratory syncytial virus (RSV)
(Adobe Stock.com)
molecular testing with LFAs. Traditional LFAs depend upon immuno-logic interactions between antigen and antibody, but in theory the process could involve nucleotides hybridizing to one another. As I mentioned before, molecular diagnostics typically involves are three steps: extraction, amplification and detection. Could you design LFAs that act as this final detection step, in combination with a simplified extraction and amplification procedures, as a way to make the sensitivity of nucleic acid testing more accessible? This is a concept investigators are actively pursuing.
What is the next ‘big thing’?
There is also the huge area – which we haven’t had much time to delve into – around the applications of nucleic acid sequencing as viral diagnostics. Those technologies and their potential clinical applications continue to emerge rapidly. But remaining on the topic of the testing already discussed here… in one sense, I think we already amidst the next big thing, in terms of greater implementation of nucleic acid amplification testing outside of central labs. It is again worth noting that the technologic drivers are not just the molecular biology or microbiology itself, but the integrated engineering. Through microfluidics, someone who isn’t necessarily a dedicated molecular biologist can inoculate a diagnostic specimen into a testing cartridge and insert that cartridge into an instrument, generating a nucleic acid amplification result in 1–2 hours or less.
This has now been coupled with multiplexing, either limited multiplexing or more comprehensive multiplexing across entire infectious syndromes – for example, respiratory viruses, gastro-intestinal pathogens, or agents of central nervous system infection. Detection in traditional qPCR is based on the fluorescent readout of individual fluorophores, so one only has so many fluorescent colours in the rainbow to work, without them all overlapping. This inherently limits the extent of multiplexing that you could do. However, syndromic molecular tests have got beyond this limitation by spatial segregation of amplicon readout. Even if even if you can’t get beyond four colours, if you can analyse one colour in dozens of different locations, you have the potential for high-level multiplexing. This really gets us to the stage where we are currently at, where – from a purely technological perspective – the ability for POC molecular testing is well established. However, there is a critical additional point to highlight there: it’s not just a matter of can you do it, but how that testing fits into the greater economic and health-system realities across the world. This goes doubly for the possibility of at-home molecular testing, around which a limited number of COVID-19 assays have been launched.
What are your final thoughts or take-home message for the future of POC or at-home diagnostics?
Maybe I’ll put my investigator hat on right now. When we look at the ability to develop new tests, one challenge we’re faced with from an R&D perspective – and this this even gets down to practical things like R&D funding – is that it’s not just developing tests based upon their analytic performance, but also this notion of ‘actionability’. Can we demonstrate attributable outcomes for a test? For drug development, outcomes is everything… but typically for lab testing, we look at test performance and then assume there’s an associated outcome. But as the technology and testing-scenarios get more and more complex, these outcomes can’t always be taken for granted. More and more, we actually have to measure/assess the outcomes that will drive clinical decision-making, to determine when new testing options are most appropriate.
Unfortunately, even if everyone agrees that we need more of these assessments, one of the challenges is determining who going to pay for outcomes-analysis? Traditionally, the manufacturer developing a test would be responsible for covering the analytic clinical trial… but should they also then fund outcomes-analysis? Ideally, that would be great, but it adds significant additional time and costs. Governmental funding could help, but publicly funded diagnostic outcomes-research is not an area that has garnered a lot of enthusiasm to date. In general, there is certainly space for the further development of POC and at-home testing. But, for any such test, we must be very clear about both the analytic question it’s addressing, and the actionable outcome of the result.
Even for the basic analytic component of diagnostic clinical trial the R&D landscape is more complicated for POC and at-home test development. If you take a traditional in-lab test, it’s a scenario where the necessary clinical trial can often be performed through specimens that are already coming into diagnostic laboratories as part of routine care. These specimens can be de-identified and you can compare old and new methodologies, validating the new test in a fairly straightforward way. Validation for POC or at-home tests becomes much more complicated. The clinicians themselves often have to be involved, or you have to go to the patients themselves to evaluate self-testing. It really makes the whole process of getting in vitro diagnostic status more difficult, because you’re not just assessing what’s going on in the lab.
The interviewee
Dr Jonathan Schmitz MD, PhD, D(ABMM),
Assistant Professor of Pathology, Microbiology and Immunology
Vanderbilt University Medical Center,
MCN T2315, Nashville, Tennessee,
37232, USA
Email: jonathan.e.schmitz@vumc.org