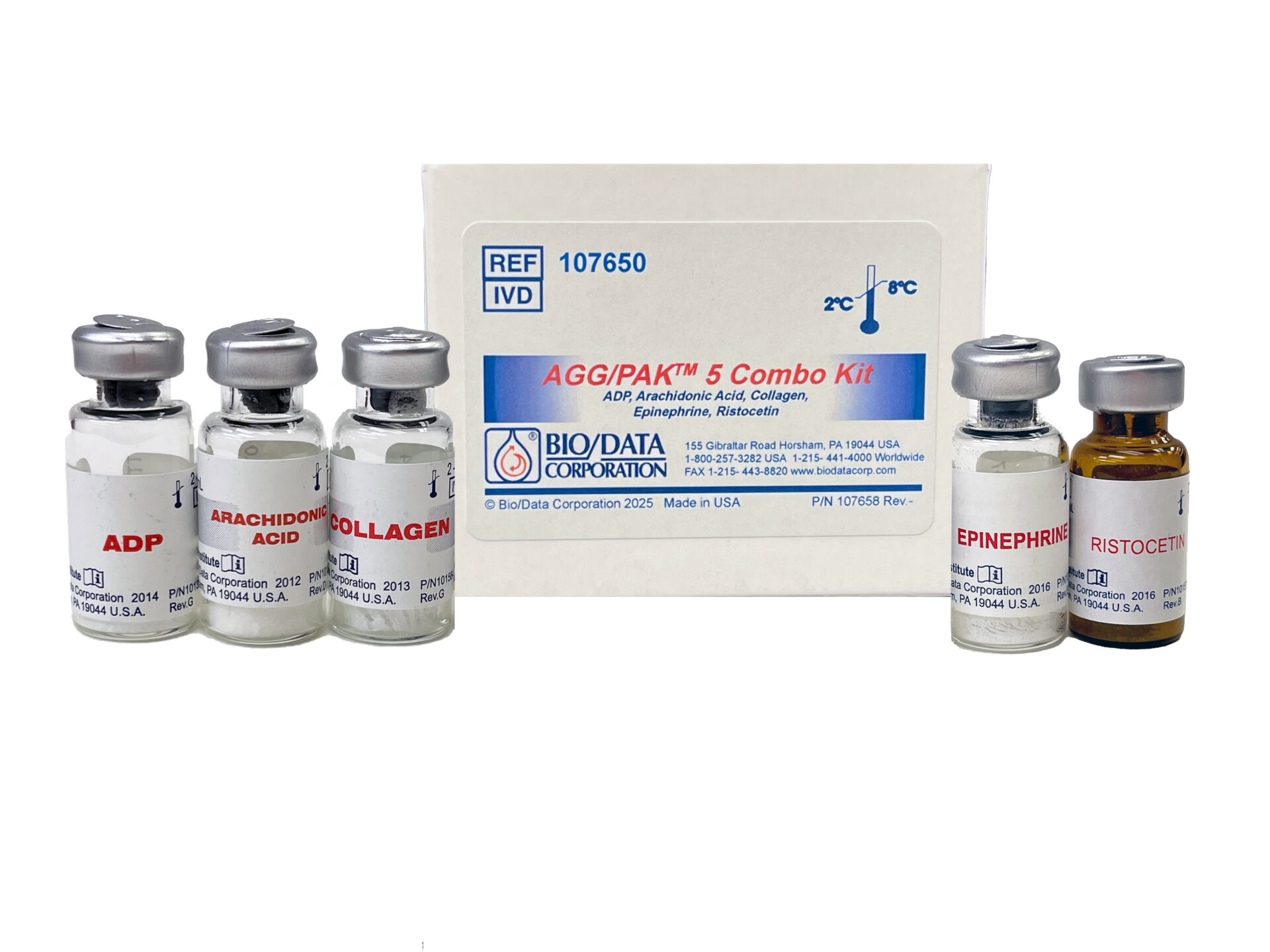
Bio/Data Corporation launches new diagnostic kit for platelet function testing
Bio/Data Corporation has introduced the AGG/PAK™ 5 Combo Kit, a comprehensive solution for platelet aggregation testing designed to enhance laboratory efficiency. The innovative kit combines five essential reagents to provide crucial insights into platelet function disorders, offering clinical laboratories improved capabilities in diagnosing bleeding disorders and monitoring anti-platelet therapies.
Comprehensive reagent combination improves diagnostic capabilities
The newly launched kit includes a strategic combination of five key reagents: ADP (Adenosine-5′-Diphosphate), Arachidonic Acid (Sodium Arachidonate), Collagen (Soluble Calf Skin, Type I), Epinephrine (Adrenaline), and Ristocetin (Ristocetin A Sulfate). This complete panel allows laboratories to conduct comprehensive platelet function assessments with standardised materials.
The inclusion of all necessary reagents in a single kit aims to streamline laboratory workflows whilst maintaining diagnostic quality. The Ristocetin component is particularly valuable for Ristocetin-Induced Platelet Aggregation Testing (RIPA), which is essential for identifying von Willebrand Disease (vWD), one of the most common inherited bleeding disorders
Applications in personalised medicine and thrombosis prevention
Beyond straightforward diagnostic applications, the AGG/PAK 5 Combo Kit offers significant utility in therapeutic monitoring. Laboratories can utilise the kit to evaluate patient responses to anti-platelet therapies, enabling clinicians to optimise treatment regimens based on individual patient responses.
The kit also provides valuable information on clot formation dynamics, potentially aiding in the early detection and prevention of thrombotic events. This dual utility in both bleeding and thrombotic disorder assessment makes the kit particularly valuable in comprehensive haemostasis laboratories.
Regulatory compliance
A critical aspect of the new product is its compliance with regulatory standards. The manufacturer notes that the AGG/PAK 5 Combo Kit meets CLIA (Clinical Laboratory Improvement Amendments) and ISTH (International Society on Thrombosis and Haemostasis) requirements, ensuring laboratories can implement the kit whilst maintaining necessary quality and compliance standards.
About the manufacturer
Bio/Data Corporation, based in Horsham, Pennsylvania, has established itself as an industry leader in platelet function testing. The company specialises in manufacturing and distributing Platelet Aggregometers, Platelet Function Centrifuges, Platelet Aggregation Reagents, Consumables, and Accessories.
The company maintains ISO 13485 registration, demonstrating its commitment to quality management systems for medical devices.
For more information, visit: www.biodatacorp.com
Digital issue: Please click here for more information