Bio-Rad launches consolidated specialty immunoassay quality control in Europe
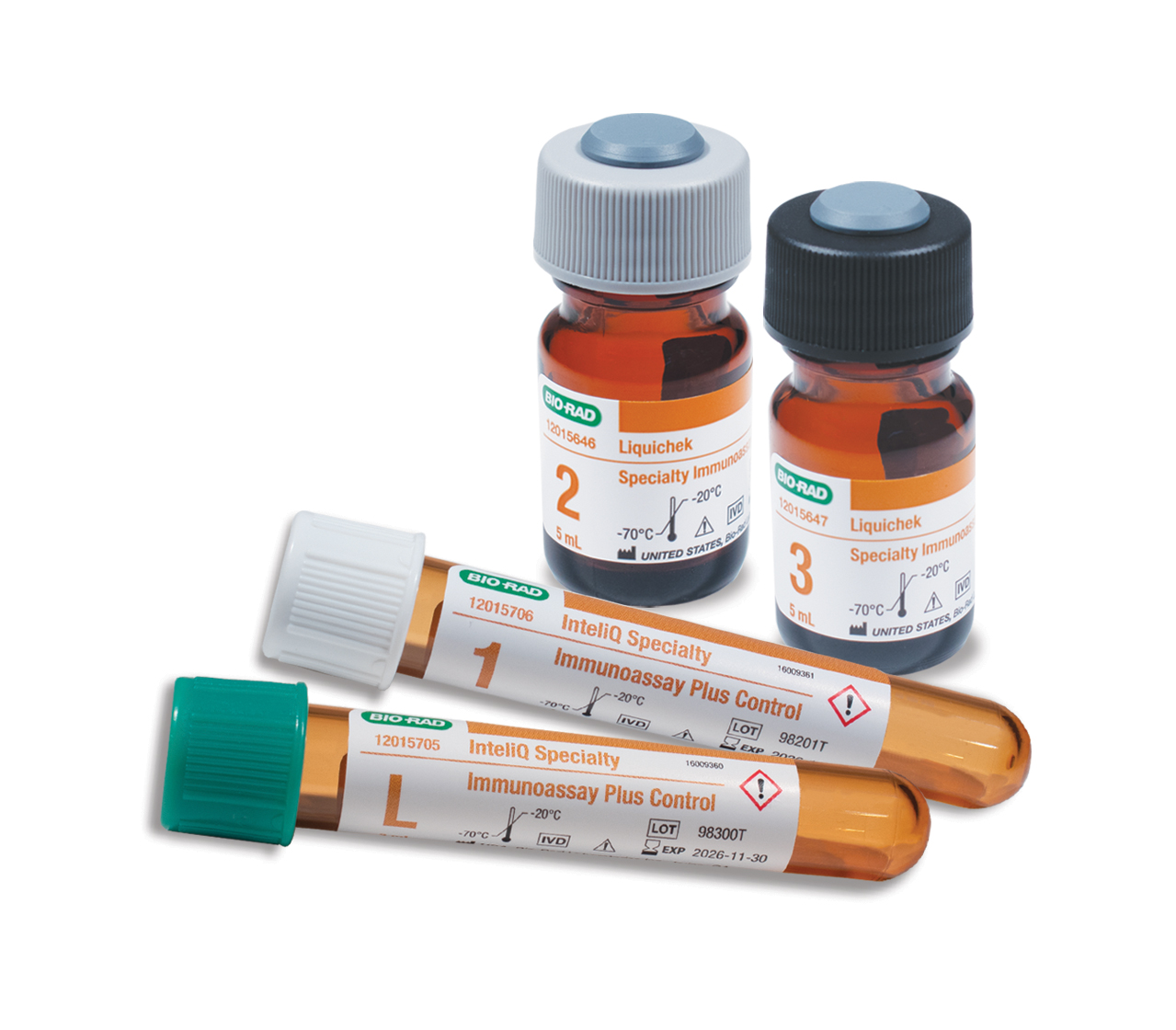
Bio-Rad launches consolidated specialty immunoassay quality control in Europe Bio-Rad Laboratories has introduced its Specialty Immunoassay Plus (SPIA Plus) control system to European clinical laboratories, offering enhanced quality monitoring capabilities for specialty immunoassay testing procedures.
Liquid-format procalcitonin integration
The four-level independent quality control system features procalcitonin in liquid format alongside high-demand analytes including IL-6, active vitamin B12, TRAb, TSI, and fructosamine. SPIA Plus incorporates optimised vitamin D ranges aligned with Vitamin D Standardization Program (VDSP) compliant assays and improved intact PTH open-vial stability designed to reduce quality control waste.
Automation-compatible configurations
Available in both barcoded InteliQ and standard vial Liquichek formats, the system offers load-and-go automation compatibility to streamline laboratory workflows. Integration with Unity data management software enables laboratories to benchmark results against a global peer group network for enhanced confidence in instrument performance and patient outcomes.
Clinical implementation
The consolidated control system addresses precision monitoring requirements across comprehensive specialty immunoassay testing protocols. The automation-ready format is designed to optimise laboratory efficiency whilst maintaining rigorousquality standards.
SPIA Plus is now available to European laboratories, offering expanded analyte coverage in a unified quality control solution.
For more information, visit: http://bit.ly/3IlsRho
Digital issue: Please click here for more information