AI framework achieves breakthrough in predicting multiple organic chemical reactions
Researchers at Wuhan University and the Shanghai Artificial Intelligence Laboratory have developed LoRA-Chem, a modular machine learning framework that accurately predicts diverse organic chemical reactions using a single unified model. The system achieves state-of-the-art performance whilst requiring only natural language descriptions of reactions as input.
Scientists have unveiled a novel approach to predicting organic chemical reactions that overcomes longstanding limitations in computational chemistry. The LoRA-Chem framework, published in CCS Chemistry, demonstrates the capacity to forecast outcomes across mechanistically distinct reaction types through a single integrated architecture – a significant advancement over conventional single-task prediction methods.
Natural language meets chemical prediction
The framework operates through a natural language interface enabled by prompt engineering, requiring only textual reaction descriptions as input. This approach represents a departure from traditional machine learning methods, which typically rely either on computationally expensive density functional theory descriptors or oversimplified molecular fingerprints.
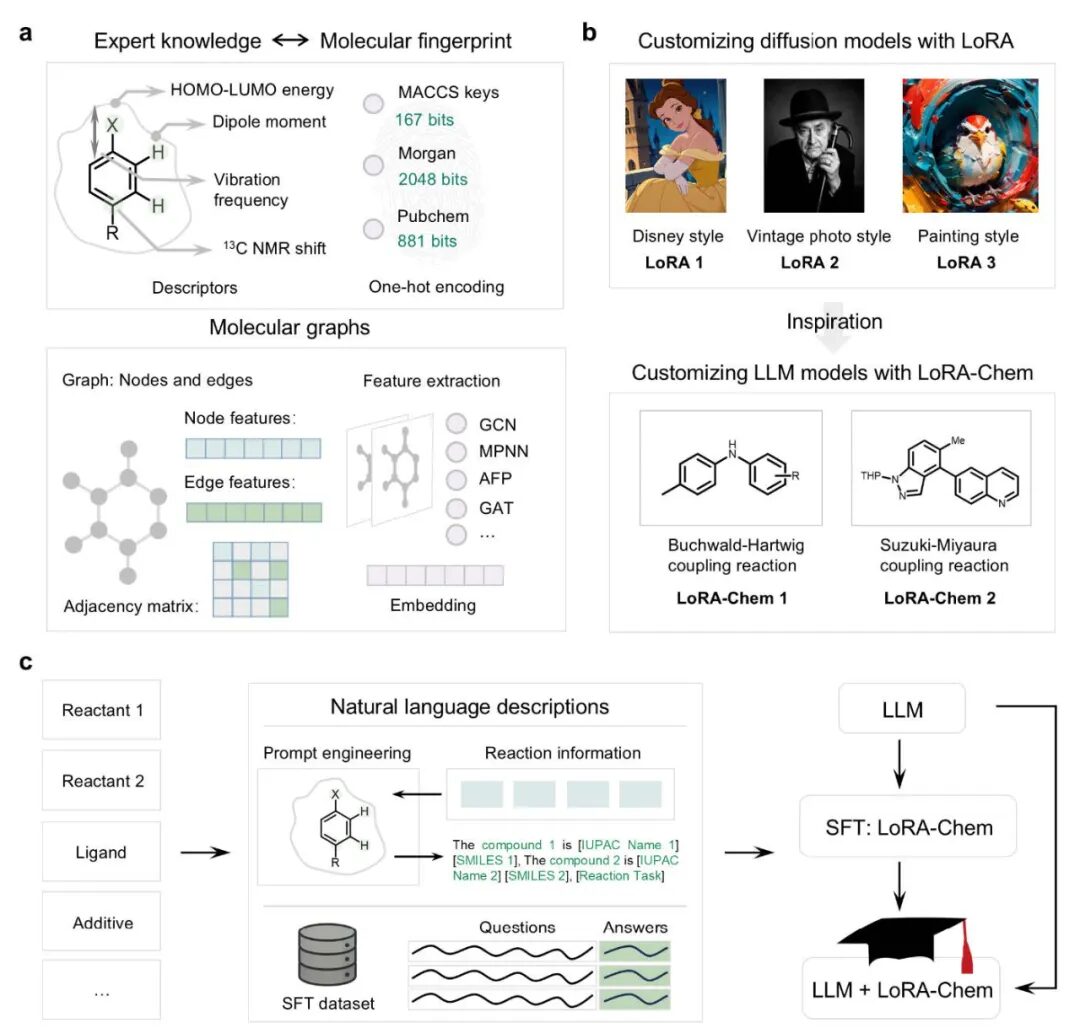
The research team, led by Professor Guoyin Yin at Wuhan University, drew inspiration from advances in AI-driven image generation. “LoRA-Chem is an innovative modular framework that demonstrates remarkable performance in predicting individual organic reactions whilst achieving unprecedented multitasking capacity,” the authors state in their paper.
The system combines a shared chemical foundation model with interchangeable low-rank adaptation (LoRA) modules, allowing specialised task adaptation for reactions such as Buchwald-Hartwig or Suzuki-Miyaura couplings whilst maintaining atomic-level chemical coherence.
Rigorous validation across reaction types
The team validated LoRA-Chem’s capabilities across multiple classical reaction datasets. In predicting selective functionalisation of sterically hindered meta-C–H bonds of o-alkylaryl ketones, the framework achieved a coefficient of determination (R²) of 0.748, representing a 1.6% improvement over previously reported values.
For Suzuki-Miyaura coupling reactions, LoRA-Chem achieved R² = 0.60 on ligand-based out-of-distribution tests. In Buchwald-Hartwig coupling reactions, the system attained R² values of 0.83 and 0.79 across two test sets, with the multitask setting reaching R² of 0.86 – establishing a new benchmark.
The framework demonstrated particular strength in enantioselectivity prediction of chiral phosphoric acid catalysis, consistently outperforming competing algorithms across multiple test sets. Notably, when tested on a dataset of 6,590 reactions with energy (ΔΔG, kcal/mol) as the predictive target, LoRA-Chem achieved R² of 0.76 and mean absolute error of 0.238 kcal/mol.
LoRA-Chem: From Model Customization to Organic Chemistry Reaction Tasks. (a) Several machine learning algorithm paradigms applied to organic chemistry. (b) Inspired by AI-driven image style customization. (c) Schematic diagram of the LoRA-Chem workflow.
© CCS Chemistry
Efficient training with modest data requirements
A key advantage of LoRA-Chem lies in its data efficiency. Unlike many deep learning systems requiring vast training datasets, the framework achieves competitive performance with only thousands of training samples. Training can be completed in approximately half a day using consumer-grade graphics processing units such as the NVIDIA GeForce RTX 4060 Ti.
The system employs a hybrid molecular representation combining both International Union of Pure and Applied Chemistry nomenclature and Simplified Molecular Input Line Entry System strings. This dual approach maximises chemical fidelity whilst maintaining compatibility with large language model pretraining.
Chain-of-thought reasoning enhances multitask learning
The researchers implemented a stepwise question-answer framework adapted from chain-of-thought prompting paradigms. This two-stage process first identifies reaction types through chemical pattern recognition before generating outcome predictions – mirroring expert chemists’ analytical workflows.
“This intermediate reasoning process forces the model to first analyse fundamental reaction characteristics before reaction results prediction,” the authors explain, noting that this approach proved essential for successful multitask learning.
Preserving base model capabilities
Critically, integration of LoRA-Chem modules preserves the original capabilities of underlying large language models. Benchmark testing across mathematical reasoning, language modelling, and multidisciplinary knowledge tasks demonstrated that models equipped with LoRA-Chem performed comparably to base models without the chemistry-specific adaptations.
The authors emphasise that performance scales with improvements in base model architecture. When fine-tuned using the Qwen2.5-7B-Instruct model, test set R² improved from 0.748 to 0.77, suggesting substantial potential for future enhancement as language models continue to advance.
Future applications in synthetic chemistry
The team has released the LoRA-Chem dataset, training code, and model files through GitHub https://github.com/flyben97/LoRA-Chem and Hugging Face https://huggingface.co/Flyben/LoRA-Chem repositories to facilitate further development by the research community.
“The demonstrated versatility and scalability of the LoRA-Chem framework suggest transformative potential for next-generation reaction prediction systems, offering a robust platform for developing sophisticated artificial intelligence solutions in synthetic chemistry,” the authors conclude.
Reference
Gao, B., Li, P., Zhang, D., et. al. (2025). LoRA-Chem: Modular machine learning for multitask prediction in organic reactions. CCS Chemistry. https://doi.org/10.31635/ccschem.025.202506542