The early risk assessment of T2DM utilizing the adiponectin assay
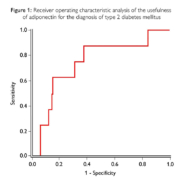
The continued rise in prevalence rates of type 2 diabetes mellitus (T2DM) and the inadequacies of the traditional T2DM risk methods highlights the necessity for superior markers for the assessment of at-risk patients. The traditional clinical tests utilized in the risk assessment of T2DM includes fasting plasma glucose (FPG) and oral glucose intolerance test (OGTT). FPG has been proven to have poor specificity and OGTT can only be detected when the underlying disease has progressed over several years. The non-biochemical methods, body mass index (BMI), employed for the classification of overweight and obese patients does not distinguish between excess fat, muscle or bone mass. Consequently, these traditional tests are inadequate for the early risk assessment and prevention of T2DM. Adiponectin is a protein hormone secreted by adipocytes with anti-inflammatory and insulin-sensitizing properties, reducing the risk of metabolic syndrome, insulin resistance, CVD and T2DM. Adiponectin levels have been found to inversely correlate with abdominal visceral fat. One study found that using the diagnostic criteria for ADA glucose and HbA1c as a reference, adiponectin displayed a sensitivity of 88% and a specificity of 51% at a cut-off point of 7.5μg/ml (figure 1). This study concluded that adiponectin levels associate with improved glycemic control, revealing its potential as a biomarker in T2DM screening and in the assessment of prediabetic state. Adiponectin levels have also been proved to be useful in the identification of gestational diabetes mellitus (GDM) risk in early pregnancy. A nested case-control study found that women with decreased adiponectin levels measured, on average, 6 years pre-pregnancy were associated with a 5-fold increased risk of developing GDM. Not only is early risk assessment of diabetes required to improve health outcomes, but also to alleviate the burden on national health services and economies. It is evident that a superior method for assessing diabetes risk is required, enabling physicians to accurately evaluate at-risk patients. Randox Laboratories are manufacturers and distributors of an automated biochemistry adiponectin test for the early risk assessment of T2DM, CVD and metabolic syndrome.