Effect of DNA extraction on molecular testing in the clinical laboratory
Extraction of nucleic acids from patient samples is an essential step for downstream molecular studies such as quantitative and qualitative PCR. The size of the DNA fragments present in samples can influence extraction efficiency, especially observed in circulating cell-free DNA (cfDNA). Further work is necessary to determine the impact of cfDNA extraction on clinical virology and microbiology testing.
by Dr Kimberly Starr and Dr Linda Cook
Introduction
After sample collection, the next important step in the detection of infectious agents in most patient-derived samples is the extraction of DNA or RNA to remove proteins, lipids, other cellular components, and PCR inhibitors to create a ‘PCR-friendly’ eluate solution. First, the sample is mixed with a lysis buffer and then DNA is purified from the resulting solution by silica-coated filtration membranes or magnetic beads that bind nucleic acid and allow subsequent washing and elution steps to be performed. Extraction methods can range from small-scale manual methods to large-scale fully-automated extraction instruments. For implementation of automated platforms several factors require consideration, including capacity, target range, efficiency, cost, physical footprint, level of automation, and processing time. The variety of instrumentation and extraction methods available contribute to the differences in extraction efficiency that may have downstream consequences when quantifying DNA or RNA in bacteria, fungi, parasites, and viruses. The performance of different kits even on the same instrument can further contribute to variation in efficiency [1]. Inter-laboratory variation as a result of extraction efficiency can affect patient care and reproducibility of testing results, especially for patients who are monitored over a long period with a quantitative test.
Extraction method comparisons
In a study comparing the bacterial DNA quantity and quality extracted from stool, Claassen et al. found DNA yield and purity varied between five commonly used extraction kits [2]. This is the case for fungi as well where extraction of nucleic acid from Aspergillus fumigatus is the main limiting factor for successful Aspergillus PCR from clinical specimens. Perry et al. found differences in reproducibility of DNA extraction at low levels (101 cells/mL) in EDTA whole blood among the four extraction instruments they tested [3]. The same can be seen in parasitic infections, demonstrated by Yera et al., which showed that DNA extraction procedures led to variations in detecting low concentrations of Toxoplasma gondii tachyzoites in amniotic fluid samples, a difference that could affect early diagnosis of congenital toxoplasmosis [4].
Other studies have evaluated extraction systems for human immunodeficiency virus (HIV) [5–8], hepatitis B virus (HBV) [9, 10], Cytomegalovirus (CMV) [11], enterovirus [12], norovirus [13], and HSV [14]. Essentially all published extraction comparison studies have seen quantitative differences in results across the different systems evaluated, sometimes with quantitative differences significantly more than 1 log.
Cell-free DNA measurements
Another level of complexity is added when the size of the nucleic acid to be isolated varies. It is known that nucleic acids fragment during the extraction process, but recent studies have demonstrated that nucleic acids may be a variety of sizes in the initial sample, especially in blood. Cell-free circulating DNA (cfDNA) in blood coming from cellular breakdown was first described by Mandel and Metais in 1948 [15]. The size of cfDNA fragments described is approximately 167 bp, equivalent to the size of chromatosome DNA and similar to post-apoptosis DNA fragments. In the last 20 years, there has been increased interest in measuring and quantifying cfDNA in a variety of cancers. Key observations from these studies are: (1) The concentration in plasma/serum is very low, 10–100 ng/mL. Thus, many studies have focused on identifying extraction methods to maximize cfDNA yield. (2) Sample collection tubes with cell-stabilizing reagents to prevent contamination of plasma with cellular DNA can increase the purity and yield of cfDNA. (3) Use of generic DNA extraction methods can cause further fragmentation of cfDNA and decrease yields compared to cfDNA-specific extraction methods. Recently, extraction instrument manufacturers have introduced cfDNA isolation kits and instruments. These kits utilize higher input volumes of 1.0–5.0 mL, and optimized temperatures or buffer conditions to improve yields. cfDNA kits from several manufacturers have been shown to have better performance in several studies. Four excellent reviews describing the technical aspects of cfDNA extraction and comparison of cfDNA extraction methods have been published [16–19].
Our DNA fragment extraction study
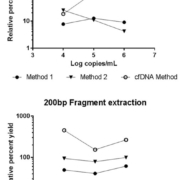
To better understand how DNA fragment size may impact viral infectious disease test results, we designed a study [20] comparing extraction yields for differently sized DNA fragments across 11 commercially available extraction methods commonly used in clinical laboratories, and also compared the performance of four new cfDNA extraction methods. Artificially constructed DNA fragments with sizes ranging from 50 to 1,500 bp were extracted and tested by droplet digital PCR to determine the DNA fragment yield across methods. We found a wide range of extraction yields across both extraction methods and instruments, with the 50 and 100 bp fragment sizes showing especially inconsistent quantitative results and poor yields of less than 20%. Figure 1 shows the yield results of two representative methods and one cfDNA method. Two of the methods designed to extract cfDNA gave the highest yields for the 50 and 100 bp fragments but overall yields were poor. We also observed the lowest variability across methods for the larger sized fragments at higher concentrations. Overall, we saw the most variability for the smallest sized fragments and observed variability dependent on concentration.
Results from our study demonstrate significant differences in fragment extraction yields and overall poor yields of the small artificial DNA fragments even at high concentrations in essentially all routinely used methods. Two of the four cfDNA methods showed improved (although still low) yield of smaller fragments. Further studies are necessary to determine the cause of this significant difference in yields. We speculate as the field moves toward more next generation sequencing approaches, these differences in extraction efficiency and quantification of small cfDNAs will become more widely described.
A critical next step is to determine if viral cfDNA exists in patients with a variety of infectious diseases and if their measurement has clinical relevance. Further studies should focus on identifying which viruses or other infectious agents have cfDNA and then methods to extract and evaluate this cfDNA must be significantly improved. To date, only cfDNA associated with Epstein-Barr virus (EBV) has been extensively studied and hints of cfDNA importance in CMV disease have been seen.
cfDNA in EBV
As early as 2003, Chan et al. described the differential detection of EBV by PCR depending on the size of the PCR amplicon, demonstrating that an assay with an 82 bp amplicon detected 7.5 times more EBV in plasma that a 181 bp amplicon assay [21]. Many additional studies in nasopharyngeal carcinoma have confirmed the excellent utility of measuring the quantity of this small EBV-associated cfDNA for monitoring of therapy response, prediction of recurrence, and monitoring at-risk populations.
Two recent large studies have shown that plasma levels of EBV are the most useful sample type for testing EBV infected patients [22, 23] but cfDNA was not specifically identified in these studies. A study by Lit et al. in EBV-associated lymphoma patients demonstrated EBV cfDNA [24] and noted that the subset of patients with ‘active’ disease had a relative predominance of cfDNA compared to predominantly larger cell-associated EBV DNA seen in cases of inactive disease or remission. Thus, measurement of both EBV cfDNA as well as larger EBV DNA fragments may be important in clinical testing and it may be necessary to distinguish the size of EBV in the plasma. Further studies are necessary to determine how useful detection of cfDNA may be in all EBV-associated malignancies and infections.
cfDNA in CMV
Published data hints that fragmented DNA may also be important for CMV PCR quantitation. In one study, Boom et al. fractionated CMV DNA in plasma and whole blood from three renal transplant cases with primary CMV infection and measured the quantities present with two PCR amplicons sized 578 bp and 134 bp [25]. They demonstrated that CMV DNA was predominantly less than 2000 bp and detected many small sized fragments only with the 134 bp amplicon PCR. Habbal et al. also studied 17 different CMV primer sets and demonstrated that the two of the four primer sets with the smallest amplicons (<100 bp) were the most sensitive for detection of cultured CMV strains [26]. Tong et al., found that among 20 solid organ transplant recipients, 10 had exclusively free CMV DNA, while the remaining 10 had predominantly free CMV DNA with a small percentage of encapsulated-virion DNA present [27]. In addition, they compared results for two assays with small amplicon sizes of 81 and 138 bp and found a 2.6-fold higher level with the smaller amplicon, suggesting CMV DNA present in these clinical samples was very small (<138 bp). It appears critical to use a high-yield small CMV DNA fragment extraction method as well as a small CMV PCR amplicon assay to maximize CMV detection of CMV. Incorporating these two elements into clinical CMV PCR assays could decrease assay variability and decrease inter-lab variability.
cfDNA in other viruses
There is evidence that cfDNA may be useful in infections and malignancies associated with viruses other than EBV and CMV. A recent study by Chesnais et al. mimicked detection of genetic mutations in pre-term children by using CCF from maternal plasma and demonstrated the potential of this technology to detect multiple viruses present in low levels in mothers or pre-term babies [28]. In addition, case reports for Kaposi’s sarcoma and BKPyV-associated bladder cancer (virus-associated cancers) suggest utility of quantitative measurements of cfDNA containing HHV8 (human herpes virus 8, also known as Kaposi’s sarcoma-associated herpesvirus) or BK virus, respectively, in tumor detection and therapeutic monitoring. Further studies are necessary in these two diseases as well as other infectious diseases to evaluate the clinical utility of cfDNA measurements.
References
1. McCulloch E, Ramage G, Jones B, Warn P, Kirkpatrick WR, Patterson TF, et al. Don’t throw your blood clots away: use of blood clot may improve sensitivity of PCR diagnosis in invasive aspergillosis. J Clin Pathol 2009; 62(6): 539–541.
2. Claassen S, du Toit E, Kaba M, Moodley C, Zar HJ, Nicol MP. A comparison of the efficiency of five different commercial DNA extraction kits for extraction of DNA from faecal samples. J Microbiol Methods 2013; 94(2): 103–110.
3. Perry MD, White PL, Barnes RA. Comparison of four automated nucleic acid extraction platforms for the recovery of DNA from Aspergillus fumigatus. J Med Microbiol 2014; 63(Pt 9): 1160–1166.
4. Yera H, Filisetti D, Bastien P, Ancelle T, Thulliez P, Delhaes L. Multicenter comparative evaluation of five commercial methods for toxoplasma DNA extraction from amniotic fluid. J Clin Microbiol 2009; 47(12): 3881–3886.
5. Cornelissen M, Gall A, Vink M, Zorgdrager F, Binter S, Edwards S, et al. From clinical sample to complete genome: comparing methods for the extraction of HIV-1 RNA for high-throughput deep sequencing. Virus Res 2017; 239: 10–16.
6. Alp A, Hascelik G. Comparison of 3 nucleic acid isolation methods for the quantification of HIV-1 RNA by Cobas Taqman real-time polymerase chain reaction system. Diagn Microbiol Infect Dis 2009; 63(4): 365–371.
7. Stevens W, Horsfield P, Scott LE. Evaluation of the performance of the automated NucliSENS easyMAG and EasyQ systems versus the Roche AmpliPrep-AMPLICOR combination for high-throughput monitoring of human immunodeficiency virus load. J Clin Microbiol 2007; 45(4): 1244–1249.
8. Swanson P, Holzmayer V, Huang S, Hay P, Adebiyi A, Rice P, et al. Performance of the automated Abbott RealTime HIV-1 assay on a genetically diverse panel of specimens from London: comparison to VERSANT HIV-1 RNA 3.0, AMPLICOR HIV-1 MONITOR v1.5, and LCx HIV RNA Quantitative assays. J Virol Methods 2006; 137(2): 184–192.
9. Kang SH, Lee EH, Park G, Jang SJ, Moon DS. Comparison of MagNA Pure 96, Chemagic MSM1, and QIAamp MinElute for hepatitis B virus nucleic acid extraction. Ann Clin Lab Sci 2012; 42(4): 370–374.
10. Pyne MT, Vest L, Clement J, Lee J, Rosvall JR, Luk K, et al. Comparison of three Roche hepatitis B virus viral load assay formats. J Clin Microbiol 2012; 50(7): 2337–2342.
11. Bravo D, Clari MA, Costa E, Munoz-Cobo B, Solano C, Jose Remigia M, et al. Comparative evaluation of three automated systems for DNA extraction in conjunction with three commercially available real-time PCR assays for quantitation of plasma Cytomegalovirus DNAemia in allogeneic stem cell transplant recipients. J Clin Microbiol 2011; 49(8): 2899–2904.
12. Shulman LM, Hindiyeh M, Muhsen K, Cohen D, Mendelson E, Sofer D. Evaluation of four different systems for extraction of RNA from stool suspensions using MS-2 coliphage as an exogenous control for RT-PCR inhibition. PLoS One 2012; 7(7): e39455.
13. Verheyen J, Kaiser R, Bozic M, Timmen-Wego M, Maier BK, Kessler HH. Extraction of viral nucleic acids: comparison of five automated nucleic acid extraction platforms. J Clin Virol 2012; 54(3): 255–259.
14. Espy MJ, Rys PN, Wold AD, Uhl JR, Sloan LM, Jenkins GD, et al. Detection of herpes simplex virus DNA in genital and dermal specimens by LightCycler PCR after extraction using the IsoQuick, MagNA Pure, and BioRobot 9604 methods. J Clin Microbiol 2001; 39(6): 2233–2236.
15. Mandel P, Metais P. Les acides nucleiques du plasma sanguin chez l’homme. C R Seances Soc Biol Fil 1948; 142(3–4): 241–243 (in French).
16. Devonshire AS, Whale AS, Gutteridge A, Jones G, Cowen S, Foy CA, et al. Towards standardisation of cell-free DNA measurement in plasma: controls for extraction efficiency, fragment size bias and quantification. Anal Bioanal Chem 2014; 406(26): 6499–6512.
17. Fong SL, Zhang JT, Lim CK, Eu KW, Liu Y. Comparison of 7 methods for extracting cell-free DNA from serum samples of colorectal cancer patients. Clin Chem 2009; 55(3): 587–589.
18. Perez-Barrios C, Nieto-Alcolado I, Torrente M, Jimenez-Sanchez C, Calvo V, Gutierrez-Sanz L, et al. Comparison of methods for circulating cell-free DNA isolation using blood from cancer patients: impact on biomarker testing. Transl Lung Cancer Res 2016; 5(6): 665–672.
19. Sorber L, Zwaenepoel K, Deschoolmeester V, Roeyen G, Lardon F, Rolfo C, et al. A comparison of cell-free DNA isolation kits: isolation and quantification of cell-free DNA in plasma. J Mol Diagn 2017; 19(1): 162–168.
20. Cook L, Starr K, Boonyaratanakornkit J, Hayden R, Caliendo AM. Does size matter? Comparison of extraction yield for different-sized DNA fragments by 7 different routine and 4 new circulating cell-free extraction methods. J Clin Microbiol 2018; 56(12): pii: e01061-18.
21. Chan KC, Zhang J, Chan AT, Lei KI, Leung SF, Chan LY, et al. Molecular characterization of circulating EBV DNA in the plasma of nasopharyngeal carcinoma and lymphoma patients. Cancer Res 2003; 63(9): 2028–2032.
22. Ruf S, Behnke-Hall K, Gruhn B, Bauer J, Horn M, Beck J, et al. Comparison of six different specimen types for Epstein-Barr viral load quantification in peripheral blood of pediatric patients after heart transplantation or after allogeneic hematopoietic stem cell transplantation. J Clin Virol 2012; 53(3): 186–194.
23. Kanakry JA, Hegde AM, Durand CM, Massie AB, Greer AE, Ambinder RF, et al. The clinical significance of EBV DNA in the plasma and peripheral blood mononuclear cells of patients with or without EBV diseases. Blood 2016; 127(16): 2007–2017.
24. Lit LC, Chan KC, Leung SF, Lei KI, Chan LY, Chow KC, et al. Distribution of cell-free and cell-associated Epstein-Barr virus (EBV) DNA in the blood of patients with nasopharyngeal carcinoma and EBV-associated lymphoma. Clin Chem 2004; 50(10): 1842–1845.
25. Boom R, Sol CJ, Schuurman T, Van Breda A, Weel JF, Beld M, et al. Human cytomegalovirus DNA in plasma and serum specimens of renal transplant recipients is highly fragmented. J Clin Microbiol 2002; 40(11): 4105–4113.
26. Habbal W, Monem F, Gartner BC. Comparative evaluation of published cytomegalovirus primers for rapid real-time PCR: which are the most sensitive? J Med Microbiol 2009; 58(Pt 7): 878–883.
27. Tong Y, Pang XL, Mabilangan C, Preiksaitis JK. Determination of the biological form of human cytomegalovirus DNA in the plasma of solid-organ transplant recipients. J Infect Dis 2017; 215(7): 1094–1101.
28. Chesnais V, Ott A, Chaplais E, Gabillard S, Pallares D, Vauloup-Fellous C, et al. Using massively parallel shotgun sequencing of maternal plasmatic cell-free DNA for cytomegalovirus DNA detection during pregnancy: a proof of concept study. Sci Rep 2018; 8(1): 4321.
The authors
Kimberly Starr1 PhD and Linda Cook*2,3 PhD, D(ABMLI)
1Clinical Microbiology Division, Department of Laboratory Medicine, University of Washington Medicine, Seattle, WA, USA
2Clinical Virology Division, Department of Laboratory Medicine, University of Washington Medicine, Seattle, WA, USA
3Vaccine and Infectious Diseases Division, Fred Hutchinson Cancer Research Center, Seattle, WA, USA
*Corresponding author
E-mail: lincook@uw.edu