Benefits of specific drugs of abuse analysis by tandem mass spectrometry in urine and oral fluid
Quantitative specific drug analysis by tandem mass spectrometry allows a wide range of drugs to be analysed in either urine or oral fluid to confirmation standards. The repertoire of drugs is based on drugs of abuse implicated in drug-related deaths in Scotland and currently includes 27 specific drugs and metabolites.
by Dr Paul Cawood and Joanne McCauley
Background
Drugs of abuse have traditionally been identified by immunoassay screening methods. Some of these are relatively non-specific and require second-line confirmatory tests, traditionally by gas chromatography–mass spectrometry (GC-MS). As drugs are not volatile this requires derivatization to render the drugs volatile. Tandem mass spectrometry (TMS) has the advantage that samples can be analysed directly without derivatization.
Drug-related deaths in Scotland are the highest in Europe and are increasing steeply [1, 2], even though the number of substance misusers has not changed recently. Most deaths are due to accidental overdosing with opiates, which causes death from heart or respiratory failure. The steep increase is the result of poly-drug use, with gabapentin/pregabalin and street benzodiazepines (such as etizolam and alprazolam) implicated in a large number of these deaths. Identification of many of these drugs is not possible by traditional immunoassay screening methods even with GC-MS confirmation. However, it is possible to identify many of these drugs by TMS.
Specific quantitative drug analysis by TMS
Urine and oral fluid drugs of abuse method
A rapid method for the analysis of drugs of abuse in urine has been reported previously [3]. This method has been modified for the analysis of drugs implicated in drug-related deaths in Scotland [2]. One transition per drug can increase the risk of false-positive results [4]; hence, each drug has two transitions and a closely matched deuterated internal standard in order to avoid these issues. Calibrators and quality control samples are made from Ceriliant certified standards. The standard set comprises morphine, codeine, 6-monoacetyl morphine (6-MAM), dihydrocodeine (DHC), oxycodone, gabapentin, pregabalin, methadone, EDDP (2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine, methadone metabolite), buprenorphine, norbuprenorphine, tramadol, amphetamine, 3,4-methylenedioxymethamphetamine (MDMA, or ecstasy) , methamphetamine, cocaine, benzoyl ecgonine (BEC), diazepam, nordiazepam, temazepam, oxazepam, 7-amino-clonazepam, nitrazepam, alprazolam, diclazepam, delorazepam and etizolam. Stock standard solution is made by adding 100 µg of each standard to a 20 ml volumetric flask, resulting in 5 000 µg/L. Calibrators are prepared at: 5, 10, 20, 30, 100, 300 and 1000 µg/L with quality controls at 10, 20, 50, 100, 300 and 400 µg/L in 3 % human serum albumin. The albumin prevents non-specific binding to the container.
Spot urine samples are collected in universal containers and oral fluid is collected into a Sarstedt salivette cortisol collection device (without preservative).
50 µL of calibrator, quality control, patient urine or oral fluid has 20 µL of zinc sulphate (0.1 mol/L) and 150 µL internal standard mixture (containing 17 deuterated internal standards – 1 µg/100 mL methanol) added. The sample is mixed and centrifuged. 75 µL of supernatant is removed and added to 300 µL of water. A volume of 20 µL is injected.
TMS analysis
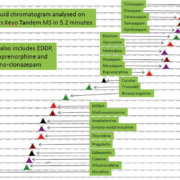
Samples are analysed on a Waters Xevo tandem mass spectrometer using a Waters Acquity ultra high performance liquid chromatography HSS C18 1.8 µm, 100 mm column at 50 °C. The sample is eluted using a multi-step gradient of water (1 % formic acid 2 mM ammonium acetate) and acetonitrile (1 % formic acid), starting at 98 % water/2 % acetonitrile to 63 % / 37 % at 3.4 min then to 5 % / 95 % at 4.5 min, reverting to 98 %/2 % at 5.2 min (Fig. 1).
Drugs are identified using the quantitative ion transition having the same peak shape as the qualitative ion transition; retention times need to match the corresponding deuterated internal standard and the quantifying ion to qualifying ion ratio matches that of the calibrators (Fig. 2). Drugs are reported as positive when above the corresponding threshold level. Threshold levels are broadly based on Driving Under the Influence of Drugs (DRUID) or European Workplace Drug Testing Society (EWDTS) confirmation test levels for both urine and oral fluid (Table 1).
We analyse 4 000 urine and 17 000 oral fluid samples each year. These are predominantly from drug problem users (Fig. 3).
Drugs of abuse in urine
TMS has the advantage of greatly reducing false-positive results seen with immunoassay methods and negating the need for second-line confirmatory tests. However, the use of urine as a sample medium still has a number of disadvantages: it is susceptible to adulteration or spiking with drugs; sample collection is not witnessed; urine drug concentrations vary depending on hydration status. This can affect whether a drug is reported as positive or negative relative to threshold levels. Additionally, some drugs are excreted relatively unchanged in urine, whereas other drugs are highly metabolized and conjugated, in which case unchanged parent drug levels can be low. In order to keep the sample preparation simple it was decided not to hydrolyse drugs in urine but to measure predominantly parent drugs, including metabolites only where necessary. This required threshold levels to be adjusted to give comparable positivity to immunoassay methods (Table 1).
Drugs of abuse in oral fluid
Oral fluid overcomes many of the disadvantages of urine: sample collection can be witnessed; samples cannot be adulterated or spiked; and threshold levels are not affected by hydration status. Since we have offered an oral fluid service most clinicians have switched from urine to oral fluid testing. Parent drugs predominate in oral fluid, with metabolite levels being generally absent or uninformative, with the exception of BEC and nordiazepam. Drugs are predominantly weak bases and diffuse from serum (pH 7.4) into oral fluid (pH 4.0–6.0). As such, some drugs are then unable to diffuse back out again. This can result in oral fluid drug levels being higher in oral fluid than in blood. Levels can remain positive for longer in oral fluid than in blood or urine, giving a longer duration of detectability for some drugs (Table 1) [5].
Opiates
Heroin contains diacetyl morphine and acetyl codeine. Both of these are rapidly metabolized into 6-MAM and codeine respectively. Both 6-MAM and codeine further metabolize to morphine. Morphine is the major excretory product of heroin in urine and is detectable in urine up to 72 h after heroin has been taken [6]. Finding 6-MAM confirms heroin has been taken. Finding codeine in the absence of 6-MAM is also compatible with codeine consumption. 6-MAM is the major heroin component in oral fluid and this always indicates heroin use. Morphine and codeine levels are generally lower than 6-MAM in oral fluid. Finding morphine in oral fluid, in the absence of 6-MAM or codeine usually indicates a pure morphine preparation has been taken. Long detection times for 6-MAM in oral fluid have been reported in a Norwegian study which analysed daily blood, urine and oral fluid samples in 20 heroin overdose cases. They reported that 6-MAM can remain positive in oral fluid for 5 days or more after heroin had been taken. In one case, the heroin test was positive 8 days after exposure [7]. Dihydrocodeine, tramadol and oxycodone can be readily identified in both urine and oral fluid.
Cocaine
Cocaine is rapidly metabolized into BEC. BEC is better than cocaine as a urine marker of cocaine use, and can be detected for 48–72 h after cocaine use [6]. However, cocaine predominates in oral fluid at much higher levels than BEC. Cocaine can remain positive in oral fluid for up to 5 days after cocaine has been taken.
Methadone/buprenorphine
Methadone and buprenorphine are prescribed for the treatment of opioid dependence and are metabolized into EDDP and norbuprenorphine, respectively. EDDP/methadone and norbuprenorphine/buprenorphine concentrations are measured in urine. Usually EDDP levels are significantly higher than methadone. Norbuprenorphine levels are usually much higher than buprenorphine. Finding methadone/buprenorphine levels greater than EDDP/norbuprenorphine indicates the sample has been spiked. Parent methadone and buprenorphine appear in oral fluid whereas EDDP and norbuprenorphine do not. Buprenorphine is administered sublingually and levels in oral fluid are very high in samples collected immediately after administration. To avoid this, oral fluid samples should not be collected within 1 h of the buprenorphine dose. Buprenorphine half-life varies from 2 to 24 h [8] and oral fluid can be negative for buprenorphine if the sample is collected the next day after a low dose.
Amphetamines
Amphetamine, MDMA and methamphetamine are excreted relative unchanged in urine. Hence, parent drugs are analysed in both urine and oral fluid.
Gabapentinoids
Gabapentin and pregabalin are predominantly excreted unchanged in urine so the parent drug is readily detected in both urine and oral fluid. A survey of substance misusers in Lothian in 2012 indicated that gabapentin was taken to potentiate the high obtained from methadone and to increase the level of intoxication [9]. 92 % of sample positive for gabapentinoids are also positive for methadone or buprenorphine confirming that these drugs are taken to boost the intoxicating effects of opiate and opioids.
Benzodiazepines
These drugs are highly metabolized and conjugated with only a small amount of parent drug excreted unchanged in urine. As such threshold levels are much lower than immunoassay screening methods. Diazepam metabolizes into nordiazepam and temazepam, both of which metabolize into oxazepam. Nordiazepam is also a metabolite of chlordiazepoxide. Finding diazepam, nordiazepam, temazepam and/or oxazepam is consistent with diazepam. Finding nordiazepam in the absence of diazepam is also consistent with chlordiazepoxide. Nordiazepam has a longer half-life than both diazepam and chlordiazepoxide and remains positive for longer than either parent drug. Detecting temazepam only, nitrazepam only or oxazepam only is consistent with those drugs being taken. These patterns persist in both urine and oral fluid, although threshold levels are lower in oral fluid compared to urine (Table 1).
Street benzodiazepines
Following the 2016 drug-related deaths Scotland report [1] we introduced testing for etizolam, delorazepam, diclazepam and alprazolam into the standard set. These drugs are generally not available by prescription in the UK. Alprazolam and etizolam are short acting, whereas delorazepam and diclazepam are long acting. Alprazolam is six times more potent than diazepam [10].
Conclusion and future developments
Gabapentinoid use is widespread and is almost always used to potentiate methadone and other opiates or opioids. There is an increasing trend for more potent street benzodiazepines. This poly-drug use has a detrimental effect on judgement and behaviour leading to inadvertent overdosing. Poly-drug use is the main reason for the increase in drug-related deaths in Scotland in recent years [2]. Identifying the main drugs implicated in these deaths is only possible by TMS. In the future, additional drugs can be considered for inclusion, such as phenazepam (30 deaths in 2017); flubromazepam (9); fentanyl (15); mirtazapine (59); amitriptyline (36); sertraline (12); fluoxetine (12); olanzapine (9); quetiapine (11) and zopiclone (29). There is evidence that these are being abused by substance misuse clients and these are all implicated in significant numbers of drug-related deaths in Scotland [11].
References
1. Drug-related deaths in Scotland in 2016. A National Statistics report for Scotland. National Records of Scotland 2017 (https://www.nrscotland.gov.uk/files//statistics/drug-related-deaths/drd2016/drug-related-deaths-16-pub.pdf).
2. Drug-related deaths in Scotland in 2017. A National Statistics report for Scotland. National Records of Scotland 2018 (https://www.nrscotland.gov.uk/files//statistics/drug-related-deaths/17/drug-related-deaths-17-pub.pdf).
3. Eichhorst JC, Etter ML, Rousseaux N, Lehotay DC. Drugs of abuse by tandem mass spectrometry: a rapid, simple method to replace immunoassays. Clin Biochem 2009; 42: 1531–1542.
4. Sauvage FL, Gaulier JM, Lachatre G, Marquet P. Pitfalls and prevention strategies for liquid chromatography-tandem mass spectrometry in selected reaction-monitoring mode for drug analysis. Clin Chem 2008; 54(9): 1519–1527.
5. Bosker WM, Huestis MA. Oral fluid testing for drugs of abuse. Clin Chem 2009; 55(11): 1910–1931.
6. Baselt RC, Cravey RH. Disposition of toxic drugs and chemicals in man. 4th edition. Chemical Toxicology Institute 1995; IBSN: 978-0962652318.
7. Baird CRW, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effects of methadone: a survey among substance misusers. Eur Addict Res 2014; 20(3): 115–118.
8. Kuhlman JJ Jr, Lanlani S, Magluilo J, Levine B, Darwin WD. Human pharmacokinetics of intravenous, sublingual and buccal buprenorphine. J Anal Toxicol 1996; 20(6): 369–378.
9. Vindenes V, Enger A, Nordal K, Johansen U, Christophersen AS, Øiestad EL. Very long detection times after high and repeated intake of heroin and methadone, measured in oral fluid. Forensic Sci 2014; 20(2): 34–41.
10. Aden GC, Thein SG Jr. Alprazolam compared to diazepam and placebo in the treatment of anxiety. J Clin Psychiatry 1980; 41(7): 245–248.
11. Barnsdale L, Gounari X, Graham L. The National Drug-Related Deaths Database (Scotland) Report. Analysis of deaths occurring in 2015 and 2016. Information Services Division, NHS National Services Scotland 2018 (https://www.isdscotland.org/Health-Topics/Drugs-and-Alcohol-Misuse/Publications/2018-06-12/2018-06-12-NDRDD-Report.pdf).
The authors
Paul Cawood* PhD
Joanne McCauley BSc
Department of Clinical Biochemistry, Royal Infirmary of Edinburgh, Edinburgh, UK
*Corresponding author
E-mail: Paul.cawood@nhs.net