Point of care and diagnosis of endometriosis
Endometriosis is a common estrogen-dependent disease affecting approximately 176 million women worldwide. Presently, a blood test for endometriosis remains elusive with laparoscopic surgery followed by histopathological confirmation of lesions remaining the gold standard for diagnosis. Women with endometriosis experience long delays between the onset of symptoms and a definitive diagnosis. The search for single or even panels of markers in the blood for the diagnosis of endometriosis has long been underway and typically met with disappointing results. Recently, plasma concentrations of brain-derived neurotrophic factor (BDNF) have been shown to have potential as a diagnostic marker of endometriosis and a novel test method was developed to enable its detection. Herein we summarize the literature suggesting a role for BDNF in the pathophysiology of endometriosis, explain why it is a promising clinical marker for this vexing condition, and introduce a point-of-care device for diagnosis.
by Dr W. G. Foster, Dr J. M. Wessles and Dr L. Soleymani
Introduction
Endometriosis is a common disease in reproductive-aged women characterized by the growth of endometrial cells anywhere in the body outside of the uterus. Epidemiological studies suggest that 6–11% of women are affected by endometriosis [1] reaching an estimated 176 million women globally. Frequent sites of endometrial disease implants include the fallopian tubes, surface of the ovaries and the space between the vagina and rectum; although implants can be found throughout the body. The cause of endometriosis remains to be elucidated; however, in some women, it is thought that during menstruation, some cells from the uterus migrate through the fallopian tubes into the pelvic cavity where they adhere to surrounding structures attach, establish a new blood supply, and grow under the influence of estrogens from the ovaries. Other potential explanations include intravasation of endometrial cells during menstruation, neonatal uterine bleeding, celomic metaplasia, immune dysfunction, and environmental factors [2].
Non-menstrual pelvic pain and infertility are common features of endometriosis that bring women with this disease to seek medical attention. Unfortunately, diagnosis has been reported to be delayed by between 6 and 12 years with an average time-to-diagnosis of 9 years from the onset of symptoms to receipt of a definitive diagnosis [3]. Hence, identification of a clinical tool for the diagnosis of endometriosis has become a high-priority research objective. Techniques in phenomics, genomics, proteomics, metabolomics and biochemistry have been employed to identify single or even panels of markers that could be exploited in the diagnosis of endometriosis. A brief overview of clinical markers is summarized below along with discussion of the data implicating brain-derived neurotrophic factor (BDNF) in the pathophysiology of endometriosis and pelvic pain leading to the suggestion of its use as a clinical marker of endometriosis.
Diagnosis of endometriosis
Healthcare providers and patients face a number of challenges in arriving at a diagnosis of endometriosis including early age at onset of symptoms, normalization of pain by primary care providers, and suppression of symptoms through intermittent use of oral contraceptive pills. Endometriosis is presumptively diagnosed through assessment of patient history, signs and symptoms, and imaging studies. However, the gold standard for diagnosis remains visualization of endometriotic lesions typically by laparoscopy followed by histopathological confirmation of disease. Unfortunately, a biochemical test for the diagnosis of endometriosis remains elusive. Multiple mechanistic pathways including dysregulation of cell adhesion, tissue remodelling, apoptosis, cell proliferation, immune function, and angiogenesis have all been explored in women with endometriosis. A plethora of biochemical differences in the peripheral circulation, peritoneal fluid, and endometrial tissues of women with endometriosis versus healthy controls have been documented [4] and explored as markers of endometriosis. For example, endometriosis induces a chronic inflammatory reaction that is characterized by alterations in interleukin-1, 6, 8, tumour necrosis factor-alpha, RANTES, and interferon gamma concentrations. However, no protein marker of endometriosis has been found to have suitable sensitivity or specificity for the diagnosis of endometriosis whether used alone or in a panel of clinical markers [4]. Consequently, the hunt for a clinical marker of endometriosis continues.
Emerging markers of interest
Emerging markers of interest for the diagnosis of endometriosis include nerve fibre density, microRNA (miRNA), and the neurotrophins. Recent studies report that nerve fibre density in the functional layer of the eutopic endometrium is greater in women with endometriosis compared to controls [5], although this conclusion was recently challenged. The measurement of nerve fibre density was suggested as a diagnostic tool for minimal to mild endometriosis (stage I and II disease). Unfortunately, measurement of nerve fibre density requires an invasive endometrial biopsy and thus is more technically demanding, painful, time consuming, and resource intensive than a simple blood test, and is therefore potentially less appealing to patients.
Recent studies have documented aberrant expression of different miRNAs in the endometrium and ectopic lesions of women with endometriosis [6]. miRNAs are short non-coding RNAs that negatively regulate mRNA translation by repressing the protein translational machinery or degrading their target transcripts. Greater than 2000 mature human miRNA sequences have been identified and are thought to regulate approximately 50% of all protein coding genes. Although widely studied in cancer, the role of miRNAs in regulation of proteins important in the pathophysiology of endometriosis is relatively unexplored. While encouraging results have been reported, replication of miRNA findings, with the exception of miR-451a, has not been demonstrated. In contrast, we suggest that complementary findings from different studies using different techniques and study populations, suggests that the neurotrophins are potentially useful clinical markers of endometriosis.
Neurotrophins and endometriosis
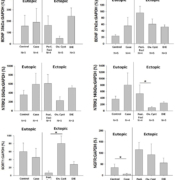
Neurotrophins of the nerve growth factor (NGF) family are soluble polypeptides that are best known for their role in neurite survival and differentiation. Neurotrophins include but are not limited to the following: NGF, BDNF, neurotrophin-3 (NT-3), and neurotrophin 4/5 (NT4/5). Although the neurotrophin family shares a common low affinity receptor, the tumour necrosis factor family neurotrophin growth factor receptor (NGFR), they also signal via high affinity neurotrophin receptors. Specifically, NGF preferentially activates neurotrophic tyrosine kinase receptor 1 (Ntrk1) whereas BDNF and NT4/5 activate Ntrk2, and NT-3 preferentially signals via Ntrk3. The neurotrophin receptors and their ligands are widely expressed in non-neuronal tissues [7] including endocrine glands [7], granulosa cells, and oocytes of fetal and adult mammalian ovaries. Furthermore, we have shown that BDNF and its receptor Ntrk2 are present in endometrial epithelial cells [8] and are expressed in the eutopic endometrium of healthy as well as the eutopic endometrium and ectopic lesions of women with endometriosis (Fig. 1). Both BDNF and Ntrk2 are localized in vascular smooth muscle and endothelial cells as well as activated macrophages and endometrial epithelium. Moreover, we have shown that BDNF can be localized to endometrial cells of ectopic lesions in women with endometriosis (Fig. 2). Hence, we suggest that BDNF and its receptor family are expressed in the endometrium and endometriotic lesions.
Previous studies have established that BDNF is synthesized as a large precursor protein (pro-BDNF) that is cleaved internally by pro-protein convertases in the trans-Golgi network and secretory granules or is cleaved extracellularly by plasmin or matrix metalloproteinases to mature BDNF (mBDNF). While pro-BDNF may be released constitutively, mBDNF is packaged in vesicles and secreted via the regulated pathway facilitated by the sorting receptor, Sortilin-I. Recently, it has been suggested that BDNF regulates divergent pathways including apoptosis, as well as differentiation and survival of discrete nerve cell populations in a receptor dependent manner. A recent proteomic study further demonstrated that BDNF and NT4/5 are both expressed in the endometrium at higher concentrations in women with endometriosis versus disease free controls [9], results that further support a role for neurotrophins as potential clinical markers of endometriosis. Thus, we suggest that the neurotrophins are potentially important in the pathophysiology of endometriosis. Specifically, similar to their roles in the central nervous system, we believe that pro-BDNF dimerizes with Sortilin-I and NGFR to promote apoptosis and inhibit macrophage infiltration whereas mBDNF binds with Ntrk2 and NGFR to facilitate resistance to apoptosis and promote cell survival, differentiation, and nerve outgrowth.
BDNF role in endometriosis and diagnosis
BDNF and the receptors Ntrk2, Sortilin-I and p75NTR are expressed in the endometrium of women (Fig. 1.) and different mammalian species [10]. A proteomic analysis of the endometrium from women with and without endometriosis revealed that BDNF protein is expressed at greater levels in the endometrium of women with endometriosis than healthy controls [9]. Using immunohistochemistry we localized BDNF to epithelial cells and blood vessels of women with endometriosis (Fig. 2). Circulating concentrations of BDNF were 2-fold greater in women with endometriosis compared to healthy fertile controls [11]. Moreover, plasma concentrations of BDNF returned to baseline levels 3 months after laparoscopic surgery to remove endometriotic lesions. We suggest that greater circulating concentrations of BDNF prior to laparoscopy followed by a decline to concentrations indistinguishable from the control population strongly implicates BDNF in the pathophysiology of endometriosis and the endometriotic lesions as a potential source of circulating BDNF. In our subsequent study [12], plasma BDNF concentrations were 1.5-fold greater in women with endometriosis compared to symptomatic controls, values that were greater in women with stage I and II disease compared to stages III and IV, suggesting a potential value of this marker in earlier stages of disease. Sensitivity and specificity of BDNF as a clinical marker of endometriosis in our laboratory has varied from 68.3–91.7% and 69.4–80.8%, respectively, depending on the population studied and the BDNF cut-off value used. Recently, although BDNF quantified in the serum was not found to be of particular value for the diagnosis of endometriosis, there was a significant correlation between serum BDNF and pelvic pain [13]. We believe this discrepancy between plasma and serum results can be explained by BDNF storage in platelets, which are lysed during blood clotting for serum collection, and might thus confound the relationship. Furthermore, since BDNF expression is estrogen regulated, stage of menstrual cycle may be important in characterizing the utility of this clinical marker in the diagnosis of endometriosis. Finally, BDNF expression in endometrial stromal cells has recently been shown to be regulated by interleukin-1β (IL-1β), an effect that is mediated through the c-Jun and NF-κB [14]. Taken together, these data suggest that the neurotrophin BDNF is expressed in the endometrium and epithelial cells of endometriotic lesions where it may contribute to neurogenesis and pain of endometriosis. Moreover, the findings of increased concentrations of BDNF in the plasma of women with endometriosis suggests potential value as a clinical marker of endometriosis.
Point-of-care diagnostic tool
Given the fact that we and others are able to detect a 1.5–2-fold difference in plasma BDNF concentrations between women with and without endometriosis, we sought to develop a clinically useful device for the diagnosis of endometriosis. Biosensors are devices that combine biorecognition with signal transduction to analyse biologically-relevant targets [15]. The glucose monitor is an example of a handheld, easy-to-use biosensor with electrochemical signal transduction used for disease management. Inspired by the widespread clinical adoption of the glucose monitor, we developed an electrochemical biosensor for diagnosing endometriosis. BDNF concentration in plasma is higher in women with endometriosis in comparison with reference populations [11, 12]. The newly developed BDNF biosensor was created using nanoporous and wrinkled electrodes [16]. These nano/microstructured electrodes enhance the sensitivity of biosensors by increasing the surface area of the transducer [17] and increasing the accessibility of the target to the biorecognition elements [18]. The specificity of the BDNF biosensor is achieved by functionalizing the nanoporous and wrinkled electrodes with anti-BDNF antibodies [19]. Signal is transduced by using an electrochemical reporter [20]. Protein, in our case BDNF, is captured at the electrode surface, and sterically hinders the access of the reporter to the electrode surface, reducing the recorded electrochemical current. As the concentration of the BDNF protein increases, the electrochemical current decreases. The decrease in electrochemical current is correlated with the concentration of BDNF measured in plasma using enzyme-linked immunosorbent assay (ELISA), the gold standard for protein analysis.
Summary
In summary, identifying a clinical marker for the diagnosis of endometriosis has been a difficult challenge. Recent evidence implicates the neurotrophin BDNF in the pathophysiology of endometriosis that is correlated with pelvic pain and potential for the diagnosis. A novel electrochemical polymer chip-based technology has been developed that can detect BDNF in human plasma and discriminate between women with and without endometriosis [16]. Combining BDNF, a novel biomarker for diagnosing endometriosis, with a sensitive electrochemical biosensor for analysing protein targets is paving the way for a diagnostic blood test for endometriosis.
Acknowledgements
The authors gratefully acknowledge the contributions of the women who have participated in our studies by providing blood and tissue samples without which our projects would not have been possible. We also gratefully acknowledge the staff of the Endo@Mac program and the clinical teams of Drs Nick Leyland, Sanjay Agarwal, Dustin Costescu and Sarah Scattolon who have enabled the tissue collection for the experiments described in this report. Annette Bullen has made our work possible through study participant recruitment and unwavering support for our efforts. The authors also are grateful for the contributions of our student Marina Bockaj without whom the endochip would not have been possible. The support of our funding partner the Canadian Institutes of Health Research (MOP142230 to WGF) is also greatly appreciated. Leyla Soleymani in the Canada Research Chair (Tier II) in Miniaturized Biomedical Devices and is supported by the Canada Research Chair program.
References
1. Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, Chen Z, Fujimoto VY, Varner MW, et al. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril 2011; 96(2): 360–365.
2. Gordts S, Koninckx P, Brosens I. Pathogenesis of deep endometriosis. Fertil Steril 2017; 108(6): 872–885 e871.
3. Ballweg ML. Impact of endometriosis on women’s health: comparative historical data show that the earlier the onset, the more severe the disease. Best Pract Res Clin Obstet Gynaecol 2004; 18(2): 201–218.
4. May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update 2011; 17(5): 637–653.
5. Tokushige N, Markham R, Russell P, Fraser IS. Different types of small nerve fibers in eutopic endometrium and myometrium in women with endometriosis. Fertil Steril 2007; 88(4): 795–803.
6. Jia SZ, Yang Y, Lang J, Sun P, Leng J. Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis. Hum Reprod 2013; 28(2): 322–330.
7. Shibayama E, Koizumi H. Cellular localization of the Trk neurotrophin receptor family in human non-neuronal tissues. Am J Pathol 1996; 148(6): 1807–1818.
8. Anger DL, Zhang B, Boutross-Tadross O, Foster WG. Tyrosine receptor kinase B (TrkB) protein expression in the human endometrium. Endocrine 2007; 31(2): 167–173.
9. Browne AS, Yu J, Huang RP, Francisco AM, Sidell N, Taylor RN. Proteomic identification of neurotrophins in the eutopic endometrium of women with endometriosis. Fertil Steril 2012; 98(3): 713–719.
10. Wessels JM, Wu L, Leyland NA, Wang H, Foster WG. The brain-uterus connection: brain derived neurotrophic factor (BDNF) and its receptor (Ntrk2) are conserved in the mammalian uterus. PLoS one 2014; 9(4): e94036.
11. Giannini A, Bucci F, Luisi S, Cela V, Pluchino N, Merlini S, Casarosa E, Russo M, Cubeddu A, et al. Brain-derived neurotrophic factor in plasma of women with endometriosis. J Endometr Pelvic Pain Disord 2018; 2(3): 144–150.
12. Wessels JM, Kay VR, Leyland NA, Agarwal SK, Foster WG. Assessing brain-derived neurotrophic factor as a novel clinical marker of endometriosis. Fertil Steril 2016; 105(1): 119–128 e115.
13. Rocha AL, Vieira EL, Ferreira MC, Maia LM, Teixeira AL, Reis FM. Plasma brain-derived neurotrophic factor in women with pelvic pain: a potential biomarker for endometriosis? Biomark Med 2017; 11(4): 313–317.
14. Yu J, Francisco AMC, Patel BG, Cline JM, Zou E, Berga SL, Taylor RN. IL-1beta stimulates brain-derived neurotrophic factor production in eutopic endometriosis stromal cell cultures: a model for cytokine regulation of neuroangiogenesis. Am J Pathol 2018;
15. Soleymani L, Li F. Mechanistic challenges and advantages of biosensor miniaturization into the nanoscale. ACS Sens 2017; 2(4): 458–467.
16. Bockaj M, Fung B, Tsoulis M, Foster WG, Soleymani L. Method for electrochemical detection of brain derived neurotrophic factor (BDNF) in plasma. Anal Chem 2018; 90(14): 8561–8566.
17. Soleymani L, Fang, L, Kelley, SO, Sargent, EH. Integrated nanostructures for direct detection of DNA at attomolar concentrations. Appl Phys Lett 2009; 95: 143701.
18. Gabardo CM, Adams-McGavin RC, Fung BC, Mahoney EJ, Fang Q, Soleymani L. Rapid prototyping of all-solution-processed multi-lengthscale electrodes using polymer-induced thin film wrinkling. Sci Rep 2017; 7: 42543.
19. Soleymani L, Fang Z, Sargent EH, Kelley SO. Programming the detection limits of biosensors through controlled nanostructuring. Nat Nanotechnol 2009; 4(12): 844–848.
20. Soleymani L, Fang Z, Lam B, Bin X, Vasilyeva E, Ross AJ, Sargent EH, Kelley SO. Hierarchical nanotextured microelectrodes overcome the molecular transport barrier to achieve rapid, direct bacterial detection. ACS Nano 2011; 5(4): 3360–3366.
The authors
Warren G. Foster*1,2 PhD, Jocelyn M. Wessles1 PhD and Leyla Soleymani2,3 PhD
1Department of Obstetrics & Gynecology, McMaster University. Hamilton, Ontario, Canada
2School of Biomedical Engineering, McMaster University, Hamilton, Ontario, Canada
3Department of Engineering Physics, McMaster University, Hamilton, Ontario, Canada
*Corresponding author
E-mail: fosterw@mcmaster.ca