The detection of anti-adrenal cortex antibodies, also known as 21-hydroxylase or 21-OH antibodies, is an aid in the diagnosis and treatment of autoimmune adrenalitis. Far from being outdated, indirect immunofluorescence is a major analytical procedure used in the autoimmune laboratory for the measurement of these autoantibodies. Several techniques can be currently adopted that allow the detection of adrenal cortex antibodies in patient serum, such as radioimmunoassays, western blot and, to a lesser extent, ELISA. Concordance of the results between immunofluorescence and immunoassays is notably good, although some discrepancies can be observed, as in many other autoimmune biomarkers.
by Dr Petraki Munujos
Autoantibody
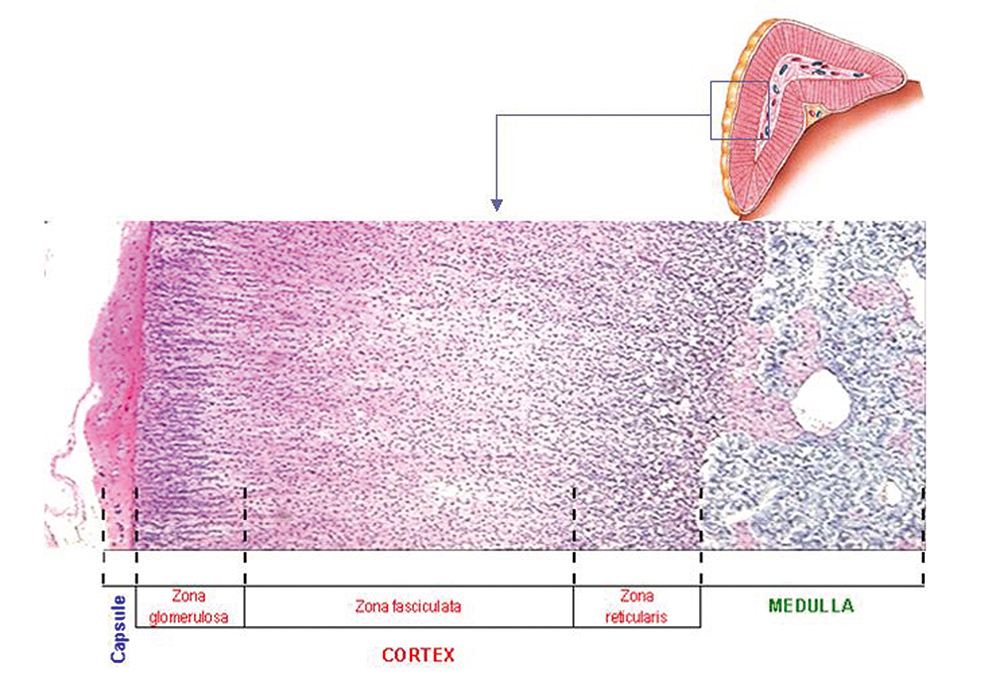
The presence of autoantibodies directed against endocrine antigens is characteristic of autoimmune polyglandular syndromes, such as Addison’s disease. Two types of autoantibodies are related to Addison’s disease: one that reacts against the cytoplasm of adrenal cortex cells (adrenal cortex autoantibodies or AACA) and another that reacts against the steroid hormones-producing cells (steroidal cell antibodies or SCA). Addison’s disease is characterized by the insuficient production of steroid hormones in the adrenal gland: mineralocorticoids like aldosterone synthesized in the zona glomerulosa, glucocorticoids like cortisol, in the zona fasciculata and androgens in the zona reticularis (Figure 1).
AACA are present in more than 90% of patients with autoimmune Addison’s disease of recent onset, and they are absent in patients with nonautoimmune Addison’s disease [1]. Whereas SCA are present in 60% to 75% of patients with autoimmune adrenal insufficiency [2]. The presence of these two autoantibodies may indicate autoimmune adrenal disease; however, diagnostic imaging studies of the gland should be performed because autoantibodies can also be found in patients with adrenal tumours or infections. Although the autoantibodies have been shown to inhibit 21-hydroxylase in vitro, it is unclear whether they play a pathogenic role or just react against the enzyme as a result of the antigen exposure after the gland injury and destruction.
Target antigen
Three autoantigens recognized by antibodies circulating in patients with Addison’s disease have been described to date. The main target antigen in Addison’s disease recognized by AACA is 21-hydroxylase, the enzyme that converts 17-α-progesterone and progesterone into 11-deoxycortisol and deoxycorticosterone (Figure 2), and it is exclusively expressed in the adrenal cortex. The main epitopes of this target autoantigen reactive with the sera from different autoimmune adrenalitis patients are located in the C-terminal and in the central region of the protein.
The other two autoantigens are recognized by SCA: 17-α-hydroxylase and P450 side-chain cleavage enzyme. These two antibodies are closely related to hypogonadism and early menopause [3,4,5].
Disease association
Addison’s disease is used as a synonym for autoimmune adrenal insufficiency or autoimune destruction of the adrenal gland. In approximately half the patients, the disease presents as an isolated autoimmune syndrome. In many patients, however, it appears as part of polyglandular endocrine failure. The main symptoms include postural hypotension, fever, abdominal pain, anorexia, nausea, weight loss, fatigue, weakness, and diarrhea. The pathologic findings are those of the infammatory processes with infiltration of lymphocytes and plasma cells.
The autoimmune adrenal insufficiency may appear isolated or it may occur in the context of the so-called autoimmune polyglandular syndrome (APS). In isolated Addison disease, AACA are detected in 65-81% of unselected patients and in 90% of those with newly diagnosed disease [6]. The prevalence of these antibodies may decrease as the disease progresses. Patients with autoimmune destruction of the adrenal gland often have other autoimmune disorders, such as hypothyroidism, Grave’s disease, vitiligo or pernicious anemia. Both Addison’s disease and premature ovarian failure are characterized by the presence of organ-specific antibodies (AACA and SCA). The main adrenal and gonadal autoantigens have been identified and cloned, and a relationship has been found between autoantibodies detected by immunofluorescence and those detected by methods that use recombinant antigens [1].
Antibodies against 21-hydroxylase can also be found in 86% of patients with autoimmune adrenalitis and 1.4% of healthy control subjects.
Methods of detection
Adrenal autoantibodies are usually analysed by indirect immunofluorescence on adrenal gland tissue sections from normal monkey. Adrenal cortex autoantibodies can be detected by immunofluorescence in 70% of cases, particularly on the glomerular zone (Figures 1, 3 and 4). Some enzyme-linked immunosorbent assays (ELISA) use recombinant human 21-hydroxylase as an antigen. Other, less common methods are Western blot, which uses recombinant antigens, and radioimmunoassay (RIA), in which the antigen is labelled with a radioligand and the procedure often includes a protein A purification step.
References
1. Betterle C, Volpato M. Adrenal and ovarian autoimmunity. Eur J Endocrinol 1998;138(1):16-25.
2. Peterson P, Uibo J, Peranen J, Krohn K. Immunoprecipitation of steroidogenic enzyme autoantigens with autoimmune polyglandular syndrome type I (APS I) sera; further evidence for independent humoral immunity to P450c17 and P450c21. Clin Exp Immunol 1997;107(2):335-40.
3. Ten S, New M, Maclaren N. Clinical review 130. Addison’s disease 2001. J Clin Endocrinol Metab 2001;86(7):2909-22.
4. Weetman AP. Autoantigens in Addison’s disease and associated syndromes. Clin Exp Immunol 1997;107(2):227-9.
5. Söderbergh A, Winqvist O, Norheim I, Rorsman F, Husebye ES, Dolva O, Karlsson FA, Kämpe O. Adrenal autoantibodies and organ-specific autoimmunity in patients with Addison’s disease. Clin Endocrinol 1996;45(4): 453-60.
6. Betterle C, Morlin L. Autoimmune Addison’s disease. Endocr Dev. 2011;20:161-72
The author
Petraki Munujos, PhD
BioSystems S.A.
Barcelona
Catalonia (Spain)