Current approaches for the detection of acute kidney injury
Acute kidney injury is a recognized complication in hospitalized patients and is associated with a high morbidity and high mortality. This brief article aims to summarize the need for early detection of acute kidney injury and the current approach within NHS England to identify such patients.
by Charlotte Fairclough
Background
Acute kidney injury (AKI) is a recognized complication in hospitalized patients. A report in 2009 from National Confidential Enquiry into Patient Outcome and Death (NCEPOD) suggested that AKI was frequently undetected in hospital patients thus contributing to patient morbidity and mortality [1]. Clinical guidelines for recognition and treatment for acute kidney injury were published by NICE (the National Institute for Health and Care Excellence) in 2013 and reported an associated mortality with AKI of more than 25–30% [2]. This guideline also recognized the prevalence of AKI in the primary care population in patients with or without acute illness. NICE also recognized the impact of AKI on healthcare resources, with costs (excluding those in the community) of £434–620 million per year, more than that associated with breast, lung and skin cancer combined [2].
AKI is characterized by an acute loss of the kidney’s excretory capacity leading to accumulation of waste products such as urea and creatinine, and decreased urine output. It is associated with rapid decline in glomerular filtration rate and increases in potassium, phosphate and hydrogen ions. It has varied causes and may be secondary to a non-renal event, thus may be common in hospitalized patients and critically ill patients. It may go undetected in primary care as it can occur without any symptoms. There are associations between co-morbidities, current medications, acute illness and AKI resulting in the high morbidity associated with the condition and the impact on healthcare resources [3].
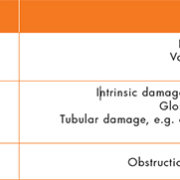
One of the most common causes of AKI is pre-renal injury due to hypovolemia (a decreased volume of circulating blood). This is thought to be the cause of more than 70% of AKI in the community [4]. This may be exacerbated in patients prescribed certain medications and should be considered carefully by primary care clinicians when assessing patients for AKI [5]. Other causes of AKI are highlighted in Table 1.
Risk factors associated with development of AKI include age, ethnicity, co-morbidities and use of certain medications [3]. It is important to detect the injury as early as possible to prevent the long-term changes in renal function that have been noted to be associated with even less-severe AKI [6].
Defining acute kidney injury
Previous definitions of acute kidney injury had been published, such as RIFLE criteria (Risk Injury, Failure, Loss, End stage renal failure) and AKIN (acute kidney injury network) [7]. KDIGO (Kidney Disease Improving Global Outcomes) published clinical practice guidance in 2011 that categorized AKI based on changes in serum creatinine and/or urine output as defined in both of these previous publications [8]. This categorized AKI into stages 1, 2 and 3 dependent on severity. Evidence suggests that even small, reversible changes in creatinine are associated with worse outcomes, and indeed AKI and severity of AKI is associated with development of chronic kidney disease [6].
The KIDIGO criteria for AKI references changes in creatinine or changes in urine output as a marker for acute kidney injury [8]. Urine output may be the functional marker of kidney function, but can be difficult to monitor. Accurate fluid balance recordings are imperative in management and prevention of AKI in a hospitalized setting, but may be difficult to do accurately especially if the patient is mobile and able to use a toilet unaided. This is also difficult to assess in community patients who obviously will not have recorded urine output as specified in the guidelines. Thus serum creatinine measurements can be used as a marker of kidney function.
Detection of acute kidney injury
Creatinine is used a biomarker for renal function because it is easy and inexpensive to measure. It is also part of most common biochemical panels in blood tests ordered in both hospital and community patients. This means it is easy to monitor trends and to compare to historical data for the patient as required for the diagnosis of AKI. But it may be slow to respond to changes in renal function, and this may be important in the early detection of AKI. Creatinine concentration in the blood and urine is also influenced by other factors such as age, muscle mass, diet, tubular secretion, hydration status and is subject to analytical interferences. Two methods for measuring creatinine are in common use in biochemistry laboratories, the traditional Jaffe methodology and enzymatic methods. Enzymatic methodology for measurement of serum creatinine has been recommended by NICE in the AKI guidelines [2]. As noted above, it has been documented that changes in creatinine only occur when 50% of kidney function has been lost. Therefore, other markers of AKI such as neutrophil gelatinase- associated lipocalin (NGAL) and tissue inhibitors of metalloproteinases- 2 (TIMP-2) have been investigated as alternatives to serum creatinine.
NGAL is a 25-kDa protein in the lipocalin family and is associated with ischaemic kidney injury and may be measured in urine. NGAL is thought to increase in the early stages of AKI as it acts to limit and repair damage caused by the insult and is mediated by NF-κB which is rapidly increased after injury and promotes cell survival and proliferation. It has been found to be detectable in urine in the very early stages of AKI [9].
Tissue inhibitor of metallinoproteinases-2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP7) have been explored as biomarkers of AKI in critically ill patients in an intensive care setting in the Sapphire study [10]. Both of these proteins are inducers of the G1 cell cycle arrest thought to be critical in the development of AKI.
The management of AKI, especially in the community is often focused on removal of the risk factors and inducers of AKI. General Practice can play a role in reduction of the risk of developing AKI such as regular review of those patients on medication associated with increased risk of development of AKI and review of patients with chronic kidney disease who are inherently at increased risk of AKI [5].
NHS England AKI detection algorithm
It was recognized that detecting AKI based on identifying changes in serum creatinine as according to KDIGO guidelines was easily automatable using laboratory information management systems (LIMS). In 2014, NHS England published a patient safety alert to all NHS Trusts with pathology services, to standardize the reporting of AKI [11]. This recognized that some Trusts had already implemented an AKI alert system based on changes in creatinine and the KDIGO guidelines, but aimed to standardize the reporting and ensure reporting was done in real-time.
The alert system algorithm is based on comparison of a patient’s creatinine concentration with that of a baseline creatinine – either a result within the last 48 hours, 7 days or 12 months based on the KDIGO criteria [12]. The patient safety alert algorithm is mandatory for all pathology laboratories in the UK and was developed with the major LIMS providers, thus enabling standardization and a model that is compatible with all systems. The mode of alerting users is not described and thus subject to differing practices within the UK NHS Trusts. This allows for laboratory interaction with users to determine the required practice for each individual Trust. For example the alerts will be reported to the electronic patient record, but whether these results are to be telephoned, emailed, etc., to users is to be individually determined. Implementation into primary care is expected to occur by April 2016 [12].
Conclusion
In summary, AKI is an important issue in healthcare due to the high level of morbidity and mortality associated with it. It is also associated with increased demand on healthcare resources throughout the system including primary and secondary care. Early detection is vital in order to reduce the morbidity and mortality associated with the condition. Every part of the healthcare system, therefore, has a part to play, including GP identification of those patients at increased risk of development of AKI and reduction of that risk, laboratory detection of AKI from serum creatinine measurements or potentially other biomarkers, and to the clinician acting on those alerts and initiating treatment early to preserve renal function.
References
1. Stewart J, Findlay G, Smith N, Kelly K, Mason M. Adding insult to injury. A review of the care of patients who died in hospital with a primary diagnosis of acute kidney injury (acute renal failure). National Confidential Enquiry into Patient Outcome and Death 2009. (http://www.ncepod.org.uk/2009report1/Downloads/AKI_summary.pdf)
2. NICE guidelines CG169. Acute kidney injury: prevention, detection and management. NICE 2013. (https://www.nice.org.uk/guidance/cg169)
3. Wang HE, Muntner P, Chertow GM, Warnock GE. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012; 35: 349–355.
4. Kaufman J, Dhakal M, Patel B, Hamburger R. Community-acquired acute renal failure. Am J Kidney Dis. 1991; 17(2): 191–198.
5. Blakeman T, Harding S, O’Donoghue D. Acute kidney injury in the community: why primary care has an important role. Br J Gen Pract. 2013; 63(609): 173–174.
6. Chawler LS, Andur R L, Amodeo RL, Kimmel PL, Palant C. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011; 79 (12): 1361–1369.
7. Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J. 2013; 6: 8–14.
8. Kidney disease: improving global outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical practice guideline for acute kidney injury. Kidney Inter. Suppl. 2012; 2: 1–138.
9. Devarajan P. Neutrophil gelatinase associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med. 2010; 4(2): 265–280.
10. Pilarczyk K, Edayadiyil-Dudasova M, Wendt D, Demircioglu E, Benedik J, Dohle DS, Jakob H, Duss F. Urinary [TIMP-2]*[IGFBP7] for early prediction of acute kidney injury after coronary artery bypass surgery. Ann intensive care 2015; 5: 50
11. Standardising the early identification of acute kidney injury. NHS England 2014. (https://www.renalreg.org/wp-content/uploads/2014/08/Patient-Safety-Alert-AKI-algorithm-2014_06_04.pdf)
12. -Acute kidney injury warning algorithm best practice guidance. NHS England and UK Renal Registry 2014. (https://www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2014/12/AKI-Warning-Algorithm-Best-PracticeGuidance-10.03.16.pdf)
The author
Charlotte Fairclough, MSc
Department of Clinical Chemistry and Metabolic Medicine, Liverpool Clinical Laboratories, Royal Liverpool and Broadgreen University Hospitals NHS Trust,
Liverpool, UK
*Corresponding author
E-mail: charlotte.fairclough@nhs.net