Circulating peptidomics: a promising approach for the diagnosis and treatment of human cancers
Recently, low-molecular-weight-peptide enrichment from blood samples by on-chip fractionation with nanopore platforms has been established successfully for the quantification and phenotypic characterization of the substrate degradome – the peptide products generated by the protease activity of a tumour environment. This article will provide evidence for this peptidomics-based approach and the clinical relevance in future therapeutic benefits will also be discussed.
by Dr Xu Qian and Dr Tony Y. Hu
Introduction
The development of cancer is a multistep process involving initiation, progression, local-regional recurrence, tumour metastasis and the host anti-tumour response. We are also now aware that changes in the broad genetic and epigenetic landscape as well as molecular mechanisms beyond histology and clinical characteristics contribute to this process. One such mechanism is the relationship between the repertoire of proteases expressed by a tissue and their substrates, which was found to be important in all steps of tumour progression by interactions with tumour cells and the tumour milieu.
Considering the systematic role of proteases in malignant tumour development, it was thought that it might be possible to detect signature products of substrate proteolysis – the substrate degradome – in the patient’s blood samples that are the result of protease dysregulation. This might then function as a diagnostic marker for tumour progression and a surrogate marker for monitoring the effects of protease-inhibitor therapy. This approach, called ‘exogenous peptidomics’ [1], based on mass spectrometry (MS) has proven its usefulness in the discovery of peptides from biofluids.
Challenges remain in this field as a consequence of the low molecular weight, low concentrations and quick degradation of such peptides in the peripheral blood of cancer patients. We recently developed an MS-based on-chip fractionation method assisted by nanopore technology, which has the advantages of being simple, high-throughput, high-resolution, and non-invasive [2]. We successfully identified circulating carboxypeptidase N (CPN)-catalysed C3f-fragments in a breast cancer mouse model as well as in patients with breast cancer [3] and matrix metalloproteinases (MMP)-9-catalysed C3f-fragments in an ovarian cancer mouse model [4]. This review discusses the applications of this new approach for studying peptide profiling in relation to tumour-resident proteases as biomarkers and potential therapy target.
Proteases and the substrate degradome in cancer development
The developing tumour microenvironment is composed of proliferating tumour cells, blood vessels, infiltrating inflammatory cells, a variety of associated tissue cells and tumour stroma, as well as secreted cytokines, chemokines, growth factors and matrix-degrading proteases. Intracellular and extracellular proteases that can function as signalling molecules play an indispensable role in this neoplastic process by enhancing cell proliferation, survival, adhesion, migration, angiogenesis, senescence, autophagy, apoptosis and evasion of the immune system in the tumour microenvironment [5–7]. For example, intracellular granzyme B is a well-known protease facilitating the ability of NK cells and CD8+ T-cells to kill their targets in the tumour milieu [8]. Elevated levels of granzyme B were also found in pre-metastatic niches presenting a novel role for the activation of CD8+ T-cells in constraining myeloid cell activity through direct killing [9]. Interestingly, recent publications suggested that granzyme B has a double-edged function. Regulatory T (Treg)-cells derived from the tumour environment may induce NK and CD8+ T-cell death in a granzyme B- and perforin-dependent fashion [10, 11] or kill CD4+ effector T-cells via granzyme B in the presence of IL-2 [12]. These findings indicate that granzyme B is relevant for Treg-cell-mediated suppression of tumour clearance in vivo.
Extracellular matrix (ECM) degradation by proteolysis is critical for tumour invasion and metastasis. Many proteases such as matrix metalloproteinases (MMPs), cathepsin and the urokinase-type plasminogen activator system play roles in the degradation of ECM in tumour progression [13, 14]. Extracellular granzyme B released from migrating cytotoxic lymphocytes was found to participate in the remodelling of vascular basement membranes (BMs) by cleaving BM constituents and enabling chemokine-driven movement through BMs in vitro [15]. Recently, another granzyme family member, granzyme M, was reported to be an inducer of epithelial-mesenchymal transition (EMT) in cancers associated with STAT3 activation [16]. Cancer cells with EMT features were capable of changing their shape, polarity and motility in a malignant manner. In the same study, overexpression of granzyme M in cancer cells was found to promote chemoresistance. The EMT phenotype of cancer cells was also achieved by increased MMP-9 production and MMP-9-mediated degradation of E-cadherin, involving ERK1/2 pathways [17].
In elucidating the role of proteases in cancer development, it is also important to gain a better understanding of the substrate degradome, which consists of the terminal peptide products of the activities of the multistage proteases. We have, therefore, identified hundreds of substrates of granzyme B, affecting cell lysis, receptors, cytokines and growth factors, as well as extracellular-matrix-structural proteins and intracellular proteins involved in cell signalling and cycle regulation [15, 18]. Besides granzyme B, MMPs cleave an increasingly large set of substrates, such as elastin, fibronectin, laminin and collagen IV [14, 19, 20]. However, given the broad range of substrate function, the mechanism of protease temporal and spatial regulation remains largely unknown. The exact role of proteases and substrates in cancer biology both at tissue level and in circulation is still needs to be clearly defined.
A new tool for the detection of circulating tumour-associated peptides as biomarkers
The proteolytic products of a protease are a useful indicator of the protease concentration in serum. This is necessary, in part, because of the low concentration of protease itself in serum compared with high detection limits, the quick degradation of the protease, and the inaccuracy of detection methodology such as enzyme linked immunosorbent assay (ELISA) as a result of irreversible non-specificity. New protocols are currently being developed for the detection and validation of substrate/peptides via peptidomics [21, 22].
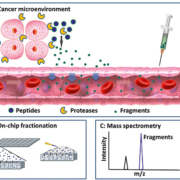
Our group developed a platform mainly including peptide on-chip fractionation followed by a matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS analysis [23]. Briefly, nanoporous silica (NPS) thin films with nanotextures are used to capture and preserve low-molecular-weight peptides from 5 μl serum or plasma samples, whereas high-molecular-weight proteins are excluded by a wash step (Fig. 1). By this on-chip fractionation, low-molecular-weight peptides are enriched and separated. The peptides appear as m/z peaks that could be detected by MALDI-TOF MS analysis. The main advantage of this platform is that it allows specific profiling of the peptidome with high-throughput, high-resolution and a simple loading step.
Using this unique platform, we tested one hypothesis that, in breast cancer, CPN activity and its proteolytic products could be detected in interstitial fluid and blood [3]. CPN together with its substrate/peptide product levels may vary during tumour initiation and progression, indicating different disease states. We confirmed by ex vivo peptide cleavage assay and in vivo validation that the previously identified substrate C3f_S1304-R1320 was cleaved by CPN specifically at the C-terminal arginine. Moreover, six fragments generated from C3f_S1304-R1320 cleavage by CPN increased significantly in mouse sera at 2 weeks after orthotopic implantation relative to normal controls. The most important finding, however, documented that the plasma levels of substrate/peptide products of CPN were apparently elevated in patients with early stage breast cancer relative to controls, but levels of CPN protein itself were unchanged. These observations indicate that there may be additional regulation of CPN at different stages of tumour development. It is likely that inhibition of CPN protease activity is included within this additional regulation. However, the presence and frequency of substrate/peptides of CPN in the early stage of breast cancer makes them potential biomarkers for early diagnosis.
Recently, we investigated MMP-9 activity with the aim of monitoring a novel therapeutic strategy. To do this we used a HeyA8-MDR-induced ovarian cancer mouse model, where HeyA8-MDR cells are a human drug-resistant ovarian cancer cell line [4]. This study provided two major observations: (1) C3f was cleaved by MMP-9 in the tumour microenvironment. Two fragments generated specifically by this proteolysis were released and were detectable in mouse serum. (2) Treatment with ephrin type-A receptor 2-siRNA-multistage vectors (MSV-EphA2) induced apoptosis of tumour cells and a down-regulation of MMP-9 in tumour tissue. Moreover, the decreased level of circulating C3f cleavage fragments correlated with MSV-EphA2 treatment. Therefore, this change could be tracked and used to monitor treatment efficiency in real-time by a simple on-chip blood test. Taken together, these data suggest that the effect of EphA2 treatment extends to the peripheral blood, well beyond the tumour microenvironment at the tissue level, and thus can be easily assessed.
We reasoned that, from these two experimental approaches, it might be possible to gain information about the dynamic processes of proteases and their substrate/peptide products in patients with cancer. Consequently, further research in this field combined with other investigations aimed at improving the management of patients with cancer by early diagnosis, accurate characterization of disease, focused, treatment efficiency, and prognosis is essential.
Conclusion
In summary, MS-based on-chip fractionation assisted by nanopore platforms has been shown to be a highly sensitive and practical tool for the quantification and characterization of the circulating degradome. The combination of cellular protease function as well as substrate/peptide analysis provides a biologically meaningful picture of a specific tumour-entity at the level of the single peptide. Analysis of the circulating pepidome will be complementary to the standard diagnostic or prognostic procedure, such as routine blood test and tissue biopsy, for patients with cancer. This approach also holds great promise as a tool for monitoring novel therapeutic targets. For further development of this technique, many predicted targets still await validation as direct protease substrates and clarification of biological relevance in the network of protease and its inhibitors. We should pay much attention to the potential pitfalls.
References
1. Peccerella T, Lukan N, Hofheinz R, Schadendorf D, Kostrezewa M, Neumaier M, Findeisen P. Endoprotease profiling with double-tagged peptide substrates: a new diagnostic approach in oncology. Clin chem. 2010; 56(2): 272–280.
2. Fan J, Niu S, Dong A, Shi J, Wu HJ, Fine DH, et al. Nanopore film based enrichment and quantification of low abundance hepcidin from human bodily fluids. Nanomedicine. 2014; 10(5): 879–888.
3. Li Y, Li Y, Chen T, Kuklina AS, Bernard P, Esteva FJ, et al. Circulating proteolytic products of carboxypeptidase N for early detection of breast cancer. Clin Chem. 2014; 60(1): 233–242.
4. Deng Z, Li Y, Fan J, Wang G, Li Y, Zhang Y, et al. Circulating peptidome to indicate the tumor-resident proteolysis. Sci Rep. 2015; 5: 9327.
5. D’Eliseo D, Pisu P, Romano C, Tubaro A, De Nunzio C, Morrone S, et al. Granzyme B is expressed in urothelial carcinoma and promotes cancer cell invasion. Int J Cancer 2010; 127(6): 1283–1294.
6. Hu SX, Wang S, Wang JP, Mills GB, Zhou Y, Xu HJ. Expression of endogenous granzyme B in a subset of human primary breast carcinomas. Br J Cancer 2003; 89(1): 135–139.
7. Jezierska A, Motyl T. Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit. 2009; 15(2): RA32–40.
8. Keefe D, Shi L, Feske S, Massol R, Navarro F, Kirchhausen T, et al. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity 2005; 23(3): 249–262.
9. Zhang W, Zhang C, Li W, Deng J, Herrmann A, Priceman SJ, et al. CD8+ T-cell immunosurveillance constrains lymphoid premetastatic myeloid cell accumulation. Eur J Immunol. 2015; 45(1): 71–81.
10. Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005; 174(4): 1783–1786.
11. Cao X, Cai SF, Fehniger TA, Song J, Collins LI, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity 2007; 27(4): 635–646.
12. Strauss L, Bergmann C, Whiteside TL. Human circulating CD4+CD25highFoxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosis. J Immunol. 2009; 182(3): 1469–1480.
13. Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004; 4(8): 617–629.
14. Ota I, Li XY, Hu Y, Weiss SJ. Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc Natl Acad Sci U S A. 2009; 106(48): 20318–2023.
15. Prakash MD, Munoz MA, Jain R, Tong PL, Koskinen A, Regner M, et al. Granzyme B promotes cytotoxic lymphocyte transmigration via basement membrane remodeling. Immunity 2014; 41(6): 960–972.
16. Wang H, Sun Q, Wu Y, Wang L, Zhou C, Ma W, et al. Granzyme M expressed by tumor cells promotes chemoresistance and EMT in vitro and metastasis in vivo associated with STAT3 activation. Oncotarget. 2015; 6(8): 5818–5831.
17. Zuo JH, Zhu W, Li MY, Li XH, Yi H, Zeng GQ, et al. Activation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT-like phenotype change and MMP-9-mediated degradation of E-cadherin. J Cell Biochem. 2011; 112(9): 2508–2517.
18. Boivin WA, Cooper DM, Hiebert PR, Granville DJ. Intracellular versus extracellular granzyme B in immunity and disease: challenging the dogma. Lab Invest. 2009; 89(11): 1195–1220.
19. Martinez A, Oh HR, Unsworth EJ, Bregonzio C, Saavedra JM, Stetler-Stevenson WG, et al. Matrix metalloproteinase-2 cleavage of adrenomedullin produces a vasoconstrictor out of a vasodilator. Biochem J. 2004; 383(Pt. 3): 413–418.
20. Wang S, Dangerfield JP, Young RE, Nourshargh S. PECAM-1, alpha6 integrins and neutrophil elastase cooperate in mediating neutrophil transmigration. J Cell Sci. 2005; 118(Pt 9): 2067–2076.
21. Kwong GA, von Maltzahn G, Murugappan G, Abudayyeh O, Mo S, Papayannopoulos IA, et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat Biotech. 2013; 31(1): 63–70.
22. Ueda K, Saichi N, Takami S, Kang D, Toyama A, Daigo Y, et al. A comprehensive peptidome profiling technology for the identification of early detection biomarkers for lung adenocarcinoma. PLoS One. 2011; 6(4): e18567.
23. Hu Y, Peng Y, Lin K, Shen H, Brousseau LC, 3rd, Sakamoto J, et al. Surface engineering on mesoporous silica chips for enriching low molecular weight phosphorylated proteins. Nanoscale 2011; 3(2): 421–428.
The authors
Xu Qian MD1,2, Tony Y. Hu PhD*1,3
1Dept of Nanomedicine, Houston Methodist Research Institute, Houston, TX 77030, USA
2Key Laboratory of Laboratory Medicine, Ministry of Education, Zhejiang Provincial Key Laboratory of Medical Genetics, Wenzhou Medical University, Zhejiang, PR China
3Dept of Cell and Developmental Biology, Weill Cornell Medical College of Cornell University, NY 10065, USA
*Corresponding author
E-mail: yhu@houstonmethodist.org