Multiplex autoantibody screening in neurological diseases
The spectrum of autoimmune neurological syndromes has expanded substantially in the last fifteen years because of the discovery of novel anti-neuronal autoantibodies. Today’s autoantibody test portfolio includes over 32 different specificities associated with neurological diseases.
Alongside classic anti-neuronal autoantibodies against intracellular targets such as Hu, Yo, Ri, etc are newly identified autoantibodies directed against cell surface antigens, for example glutamate receptors of type NMDA or AMPA, GABAB receptors, the voltage-gated potassium channel-associated proteins LGI1 and CASPR2, or DPPX. Since many of the autoantibodies occur only rarely, multiplex screening is favoured over selective or sequential testing to avoid diagnostic gaps. Anti-neuronal autoantibodies can be efficiently screened using multiplex indirect immunofluorescence tests (IIFT) comprising mosaics of tissue sections and monospecific recombinant-cell substrates. Immunoblots containing extensive panels of purified antigens are ideal for confirming antibody specificities.
Paraneoplastic neurological syndromes
Paraneoplastic neurological syndromes (PNS) are disorders of the central and peripheral nervous system that occur in direct relation to tumour development. However, they are not caused directly by the tumour or its metastases or by therapeutic side effects. The cells of the tumour express antigens that normally only occur in neurons. These induce the formation of specific antibodies, which then bind to the corresponding antigens in the nervous tissue and damage the neurons. PNS develop in around 15% of malignant diseases, occurring most frequently in small-cell lung carcinoma, neuroblastoma, thymoma, and cancers of the ovary, mamma, uterus and testis.
Stiff-person syndrome
Stiff-person syndrome is a rare neurological disease which can be paraneoplastic or non-paraneoplastic in origin. The disease manifests with severe progressive muscle stiffness, typically in the spine and lower extremities. Paraneoplastic cases are associated with antibodies against amphiphysin. Non-paraneoplastic cases are characterized by autoantibodies against glutamate acid decarboxylase (GAD), which are found in 60-90% of patients. However, anti-GAD antibodies are not specific markers for stiff-person syndrome as they also occur in other neurological diseases and, in particular, in diabetes mellitus type I.
Autoimmune limbic encephalitis
Autoimmune limbic encephalitis encompasses a range of disorders manifesting with memory deficits, neuropsychiatric symptoms and epileptic seizures. A few years ago this condition was primarily attributed to classic paraneoplastic antibodies. However, in recent years several novel autoantibodies have been described as being associated with this condition. They differ from classic antibodies in that they are directed against target antigens on the cell surface of neurons, typically canal or receptor proteins. In some cases the autoantibodies are associated with malignancies, but in many cases no tumour is detected. Thus, the associated encephalitides are classified as facultative PNS. The autoantibodies are ascribed a direct pathogenic role. Binding of the autoantibodies to the corresponding membrane proteins interferes with synaptic signal transduction, resulting in neuropsychiatric deficits. A favourable prognosis for autoimmune limbic encephalitides is highly dependent on early diagnosis and intervention. The immediate initiation of immunotherapy and, in paraneoplastic cases, tumour removal helps to stabilize the patients and improve their overall outlook.
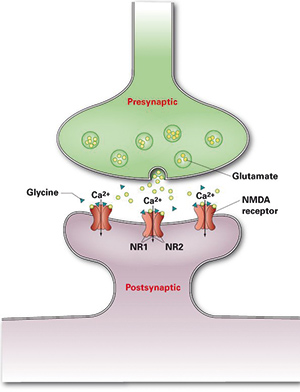
Cell surface antigens that have been recently characterized as targets of autoantibodies in limbic encephalitis include glutamate receptors of types NMDA (Figure 1) and AMPA, GABAB receptors, LGI1, CASPR2, and DPPX.
Anti-NMDA receptor encephalitis
Anti-NMDA receptor encephalitis is an inflammatory encephalopathic autoimmune disease, which is characterized by highly specific autoantibodies against glutamate receptors of type NMDA (N-methyl-D-aspartate). The disease was first described in 2007 and is currently still widely underdiagnosed. It frequently affects young women with ovarian teratoma, but is also observed in older female patients, in women without tumours, in men and in children. Paraneoplastic cases represent 9-55% of the total, depending on age, gender and ethnicity. Patients usually present with symptoms such as memory loss, disorientation, confusion, paranoid thoughts, visual or auditory hallucinations, dyskinesias, decrease in consciousness, lethargy, seizures and autonomic instability.
Detection of anti-glutamate receptor (type NMDA) autoantibodies constitutes a key criterion for the diagnosis of anti-NMDA receptor encephalitis. Their analysis is particularly important for differential diagnosis in patients with encephalitis of unknown origin, i.e. non-infectious etiology, and in young women with de novo epilepsy. When a positive serological result is obtained, a comprehensive teratoma investigation should be undertaken.
Anti-AMPAR, GABABR and LGI1/CASPR2/DPPX encephalitides
Limbic encephalitides triggered by autoantibodies against glutamate receptors of type AMPA, GABAB receptors, LGI1, CASPR2 or DPPX occur less frequently than anti-NMDA receptor encephalitis. The different disease subtypes manifest with varying symptom complexes, encompassing memory deficits, seizures, confusion, disorientation, neuromyotonia, agitation, behavioural problems, hallucinations, paranoia, hyponatremia, myoclonus, dysautonomia and sleep or consciousness disturbances. Tumours are found with differing frequencies, more commonly in patients exhibiting anti-AMPA (70%) or anti-GABAB receptor (60%) antibodies than in individuals with anti-LGI1 (<20%) or anti-CASPR2 (<20%) positivity. Limbic encephalitis associated with anti-glutamate receptor (type AMPA) antibodies occurs predominantly in women, while the anti-GABAB receptor and LGI1/CASPR2 subtypes are found more frequently in men. Anti-CASPR2 antibodies are also observed in acquired neuromyotonia and Morvan’s syndrome.
Neuromyelitis optica
The inflammatory autoimmune disease neuromyelitis optica (NMO) is a rare form of the group of acquired demyelinating diseases of the central nervous system. It is characterized by degradation of the insulating sheath of at least one optical nerve and at the same time or a few months later the spinal cord. Symptoms encompass acute visual disorders including blindness, impaired mobility and loss of bladder and bowel control. Without adequate therapy, half of patients become blind in one or both eyes or cannot walk without supports within five years.
Highly specific autoantibodies are found frequently in NMO. The antibodies were first described as NMO-IgG. The protein aquaporin-4 (AQP-4) was later identified as the target antigen. The determination of anti-AQP-4 antibodies is particularly useful for serologically differentiating NMO from classic multiple sclerosis.
Autoantibody detection methods
According to the European Network for Paraneoplastic Neurological Diseases (PNS Euronetwork), antibodies in PNS should always be detected using two unrelated laboratory methods, for example IIFT and immunoblot. The tissue substrates cerebellum, hippocampus, nerve and intestine are used in IIFT for detection of PNS antibodies. Characteristic immunofluorescence patterns indicate the presence of particular autoantibodies (Figure 2). For example, anti-Hu antibodies show granular fluorescence of the neuronal nuclei on cerebellum and hippocampus. With anti-Yo antibodies the Purkinje cells of the cerebellum and to some extent the hippocampus fluoresce. Anti-DNER antibodies can be detected monospecifically using a recombinant-cell substrate of transfected human cells.
The autoantibody specificities obtained in IIFT can be confirmed using line blots containing purified, characterized recombinant antigens. Since the antigens are located at defined positions on the membrane, these blots are extremely easy to evaluate. One of the most extensive panels of neuronal antigens is provided by a new EUROLINE Profile (Figure 3) comprising the twelve antigens: amphiphysin, CV2, Ma2/Ta, Ri, Yo, Hu, recoverin, SOX1, titin, Zic4, GAD65 and Tr (DNER). With this profile, a comprehensive confirmation of anti-neuronal antibodies can be achieved in one simple test.
Autoantibodies against neuronal surface antigens in limbic encephalitis can be detected monospecifically using recombinant-cell IIFT substrates, which consist of transfected human cells expressing defined, well-characterized whole target antigens or the immunoreactive subunits thereof. These tests offer high sensitivity and are easy to evaluate. A further advantage of recombinant-cell IIFT systems is that they can be developed in a short time, often in only a few months. Thus, newly identified autoantibody parameters can progress rapidly from the research laboratory into routine diagnostics. Recombinant-cell substrates are available for the detection of autoantibodies against, for example, glutamate receptors of types NMDA (Figure 4) and AMPA, GABAB receptors, LGI1, CASPR2, DPPX and AQP-4. The efficacy of recombinant-cell assays has been confirmed in various clinical studies. For example, the anti-glutamate receptor (type NMDA) IIFT demonstrated 100% clinical sensitivity and specificity for anti-NMDA receptor encephalitis, while the anti-AQP-4 IIFT yielded a sensitivity of 80% for NMO with a specificity of 100%.
Multiparametric IIFT screening
Since autoimmune neurological syndromes often present with overlapping symptom complexes making them difficult to diagnose, the autoantibody screening should be as wide ranging as possible (1). With IIFT BIOCHIP Mosaics (Figure 5), different tissue and recombinant-cell substrates can be analysed simultaneously. Miniature sections of the substrates are positioned next to each other in each test field of a microscope slide and incubated in parallel with one patient sample. The BIOCHIP slides are incubated using the established Titerplane technique, which provides standardized, parallel incubation of multiple samples under identical conditions. The procedure is thus fast and reliable and is suitable for use in any laboratory familiar with immunofluorescence. Automation options are available to further boost the efficiency and convenience of the analyses.
Clinical studies
The importance of multiparametric screening is highlighted by studies on sera sent to a routine clinical immunology laboratory for analysis of anti-neuronal autoantibodies. The sera were subjected to multiplex testing regardless of the test order. In many cases antibody specificities other than those expected were detected.
In a study encompassing 2716 samples submitted over a three-month period (2), 4% were found to be positive for anti-neuronal autoantibodies. Of the positive sera, 67% exhibited positivity for autoantibodies against neuronal surface antigens, compared to 35% for classic intracellular onconeural autoantibodies. Thus, neuronal surface antibodies appear to be more common than classic paraneoplastic autoantibodies. In 31% of the findings, the positive reactions were caused by an autoantibody other than that requested for testing. The antibody frequencies in descending order were NMDAR (42%), CASPR2 (12%), LGI1 (11%), Hu (10%), Ri (9%), Yo (8%), CV2 (6%), GABABR (4%), Ma (2%), PCA-2 (1%) and amphiphysin (1%).
In a second study of 16,741 sera submitted over a time period of one year (3), 14% showed at least one anti-neural autoantibody. From the positive sera that had been submitted for only one monospecific antibody test, 56% contained the requested antibody, while 49% showed a different relevant antibody (5.2% as secondary finding). A wide range of anti-neural antibody specificities was detected. For cell-surface autoantibodies the most common were NMDAR (26%), AQP4 (14%), LGI1 (7%), CASPR2 (7%), GABABR (2%), AMPAR, DPPX, GlyR, MOG, other (1% each), while intracellular autoantibodies were mainly directed against Hu (11%), Yo (10%), GAD (8%), amphiphysin, Ri (2% each), CV2, Ma/Ta, recoverin, Sox1, titin, other (1% each).
Perspectives
Autoantibody testing is an important and increasingly relevant tool for neurologists thanks to the ever expanding portfolio of neuronal autoantibodies. More cases of autoimmune neurological disease are now diagnosed at an early stage, boosting the chances of a favourable outcome for the patients. Of particularly importance is the use of autoantibody parameters as a harbinger of tumour disease. Positivity in neuronal autoantibody tests helps to direct diagnostic efforts towards searching for a neoplasm. In patients with previous cancer, the presence of autoantibodies may signal a return of the malignancy. Patients with non-paraneoplastic autoimmune disorders also benefit from early diagnosis with prompt immunotherapy. Multiparametric assays provide the greatest efficacy in diagnostic screening, as they identify not just the expected parameters, but also secondary reactivities. It is anticipated that ongoing research will identify more relevant anti-neuronal autoantibodies in the future, enabling the diagnostic net to be extended still further.
References
1. Probst et al. Multiple Sclerosis and Related Disorders 2014: 3 (3): 303-320.
2. Wandinger et al. J. Lab. Med. 2012: 35: 329-42.
3. Komorowski et al. 66th AAN Annual Meeting (Philadelphia, USA) 2014
The author
Jacqueline Gosink, PhD
EUROIMMUN AG
Seekamp 31
23560 Luebeck
Germany
E-Mail: j.gosink@euroimmun.de