Measurement traceability of Mindray CL-2000i Chemiluminescence Immunoassay System
Establishing metrological traceability of measurement is essential to improve the accuracy and comparability of measurement results. With increasing recognition of the importance of traceability, some regulatory policies have been applied to enforce its implementation. Technology advancement also provides more tools for improving measurement traceability. During the assay development on the Mindray CL-2000i Chemiluminescence Immunoassay System, well recognized highest reference methods or reference materials were used in assigning the values of master calibrators; the accuracy of product calibrators was guaranteed through an unbroken metrological traceability chain.
by Xiang Yu and Ke Li
Introduction
With the advancement in automation over the past 20 years, most of the immunoassays have been shifted from traditional manual assays to fully automatic systems leading to an overall improvement of the quality of measurements. The accuracy and comparability of testing results have been emphasized, since they are the keys to defining and using common clinical decision values and reference intervals, following constant standards and practice guidelines, pooling data from different studies based on different analytical systems to facilitate clinical research.
One critical mechanism to improve the accuracy and comparability of clinical testing results is to make the testing results traceable to higher reference materials or methods in calibration hierarchy. Briefly, the testing results should have metrological traceability. The general principles and features have been described in the document of the International Organization for Standardization (ISO) 17511:2003 [1].
Ideally, results produced by different routine methods for the same measurand should be metrologically traceable to the highest level of calibration hierarchy – the International System of Units (SI units), with an estimated measurement uncertainty. However, only a limited number of analytes, including some metabolites, electrolytes, steroid hormones, has reference materials available with traceability to the SI unit. Most of the clinical analytes still have no primary and secondary reference measurement procedures and are not traceable to the SI unit. They are not well defined and have only traceability to an international conventional standard or manufacturers’ internal standard, such as tumour markers and viral antigens [2].
The EU directive on in vitro diagnostic devices (IVDD) enacted in 1998 stated “The traceability of values assigned to calibrators and/or control materials must be assured through available reference measurement procedures and/or available reference materials of a higher order” [3]. Therefore, for all the IVD analytical system (reagents), manufacturers must ensure their products are standardized against available reference materials or methods in order to be distributed in the EU market.
Traceability chain and value assignment procedure on Mindray CL-2000i System
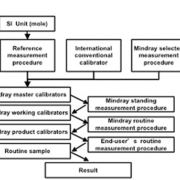
Mindray CL-2000i system is a closed system composed of a fully automatic immunoanalyser, related reagents and calibrators. The calibration hierarchy was established and documented strictly based on EN ISO 17511:2003 [1]. Mindray’s traceability procedure is indicated in figure 1, ensuring the establishment of metrological traceability between the testing results and the highest standard available. Based on the characteristics of different analytes, three major traceability chains have been used: traceable to an SI unit, traceable to an international conventional calibrator, and traceable to manufacturers’ selected procedure.
Measurements traceable to the SI unit
If the chemical and physical properties of an analyte are well defined, there should be a primary reference measurement procedure with the measurement traceable to the SI unit (mole). CL-2000i total T3, total T4, progesterone, testosterone and estradiol are traceable to this highest level of calibration hierarchy. Mindray has performed the traceability of the above measurements in collaboration with the Reference Institute for Bioanalytics (RfB), a German reference laboratory certified by the Joint Committee for Traceability in Laboratory Medicine (JCTLM) [4]. Thirty Mindray master calibrators at different levels covering the whole detection range were assigned values for each analyte at RfB with the reference measurement procedure of Isotope dilution mass spectrometry (ID-MS). The calibrator values with uncertainty were then applied to define the values of Mindray working calibrators and product calibrators, and the metrological traceability between the testing results of CL-2000i end-users’ routine measurement procedure and the SI unit was finally established. The assays that are traceable to the SI unit are indicated in Table 1.
Measurements traceable to an international conventional calibrator
The reference materials, such as WHO standards and some national standard materials are defined by convention or consensus, without traceability to the SI unit; the assigned values are in arbitrary units (e.g. WHO international unit). Most of assays for tumour markers, hormones, and viral antigen/antibody of the CL-2000i system are traceable to this kind of reference materials, indicated in Table 1.
Measurements traceable to manufacturers’ selected procedure
For analytes that are either not traceable to the SI unit, or for which no reference method and reference material are available, a commercial certified measurement procedure with traceability, high accuracy and analytical specificity was selected for Mindray master calibrator value assignment; the measurement accuracy of the Mindray routine measurement procedure is ensured and also indicated in Table 1.
Principle of the traceability of Mindray CL-2000i end-user’s measurement results
The immunoanalyser is calibrated by measuring three levels of product calibrators and relative light units (RLUs) generated. The corresponding concentration of each calibrator was used to adjust the master calibration curve stored in the barcode of each lot of reagents.
The value of end-user’s product calibrators and the master curve stored in the barcode are both defined by the Mindray routine measurement procedure that is calibrated by Mindray working calibrators in the manufacturer’s laboratory. The working calibrators have roughly 12 concentration levels and have the same matrix as the end-user’s product calibrators.
It is the Mindray standard measurement procedure that determines the values of Mindray working calibrators. The Mindray standard measurement procedure makes use of the Mindray standard CL-2000i automatic immunoassay analyser, standard reagents, and Mindray master calibrators. Mindray master calibrators are composed of a series of human serum at different concentration levels. They are stored at -70°C and represent the highest accepted standard available.
The values of the Mindray master calibrators are fixed, and the measurement standard established by the Mindray standard measurement procedure is preferably not variable and should be kept as consistent as possible. On the other hand, the value of working calibrators and end-user’s product calibrators can be flexible within a certain range. The assigned values of calibrators will be adjusted according to the results of internal QC and method comparison so as to ensure the traceability between the reference and end-user’s routine measurement procedures.
Discussion
We have made our best efforts for the traceability of the Mindray CL-2000i system, eventhough the implementation of traceability is challenging, especially the traceability in immunoassays.
Firstly, majority of analytes lack a primary reference measurement procedure and thus are not traceable to the SI units. The chemistry and physical properties of these analytes still require more accurate definition.
Secondly, the international conventional calibrators have played an important role in harmonizing testing results. However, there are still some issues with using the international standards, such as the long term stability of WHO standards, the matrix effect, the difference between different generations of the standards, and difference between the source of the standards and the real sample in the clinic.
Thirdly, some of the analytes have neither reference materials nor reference methods available, and are only traceable to manufacturers’ in-house standards. The harmonization of clinical results could not be fully implemented [5].
References
1. ISO 17511:2003. In vitro diagnostic medical devices –measurement of quantities in biological samples – metrological traceability of values assigned to calibrators and control materials. Geneva, Switzerland: ISO
2. Database of higher-order reference materials and reference measurement methods/procedures. http://www.bipm.org/en/committees/jc/jctlm/jctlm-db
3. Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. Off J Eur Union 7 December 1998; L 331:1–37.
4. JCTLM: Joint Committee for Traceability in Laboratory Medicine. http://www.bipm.org/en/committees/jc/jctlm/
5. Danni L. Meany and Daniel W. Chan Comparability of tumor marker immunoassays: still an important issue for clinical diagnostics? Clin Chem Lab Med 2008; 46(5):575–576.
The authors
Xiang Yu*, MSc and Ke Li, PhD
Immunoassay Department, Shenzhen
Mindray Bio-Medical Electronics Co. Ltd., Nanshan, Shenzhen, 518057 China
*Corresponding author
Email: yuxiang@mindray.com